Stiripentol inhibits spike-and-wave discharges in animal models of absence seizures: A new mechanism of action involving T-type calcium channels
- PMID: 35184274
- PMCID: PMC9314114
- DOI: 10.1111/epi.17201
Stiripentol inhibits spike-and-wave discharges in animal models of absence seizures: A new mechanism of action involving T-type calcium channels
Abstract
Objective: Stiripentol (STP; Diacomit®) is an antiepileptic drug indicated for Dravet syndrome that has been identified as a γ-aminobutyric acid (GABAergic) positive allosteric modulator. Dravet syndrome is characterized by multiple seizure types: generalized tonic-clonic, focal, myoclonic, and absence seizures. In addition to its antiepileptic effects on tonic-clonic seizures, STP has also been reported to reduce the frequency of atypical absence seizures in patients. Our study focused on STP potential effects on absence seizures, to better characterize its full spectrum of mechanisms of action.
Methods: STP effects on absence seizures were quantified by electroencephalographic recording in two animal models: rats treated with a low dose of pentylenetetrazol (20 mg/kg ip) and rats from the WAG/Rij strain. In addition, we characterized STP effects on T-type calcium channel activity. Peak currents were recorded with manual patch clamp on cells transfected with cDNA encoding for the human isoform for Cav 3.1, Cav 3.2, and Cav 3.3.
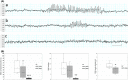
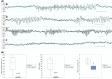
Results: STP administered before pentylenetetrazol almost completely abolished the generation of spike-and-wave discharges (SWDs) at the dose of 300 mg/kg. At this dose, STP also statistically significantly decreased SWD cumulated duration and number in WAG/Rij rats. Its antiepileptic effect was maintained in WAG/Rij rats, whose seizures were aggravated by the GABA agonist THIP (gaboxadol hydrochloride). Furthermore, electrophysiological recordings showed that STP inhibits T-type calcium channel peak activity, with a higher specificity for the Cav 3.3 subtype.
Significance: In addition to its previously characterized anticonvulsive properties, these data highlight a new mechanism of action of STP on abnormal thalamocortical activity. This strong antiabsence effect on seizures is correlated with an inhibition of T-type calcium channels. This new mechanism of action could be implicated in the specificity of STP therapeutic effects in Dravet syndrome.
Keywords: Dravet syndrome; absence seizures; epilepsy; spike-and-wave discharges; stiripentol; thalamocortical oscillations.
© 2022 The Authors. Epilepsia published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
V.R., I.H., L.R., and M.V. are full‐time employees at Biocodex. H.S.H. has no conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures


Similar articles
-
An Update on Stiripentol Mechanisms of Action: A Narrative Review.Adv Ther. 2024 Apr;41(4):1351-1371. doi: 10.1007/s12325-024-02813-0. Epub 2024 Mar 5. Adv Ther. 2024. PMID: 38443647 Free PMC article. Review.
-
The role of HCN channels on the effects of T-type calcium channels and GABAA receptors in the absence epilepsy model of WAG/Rij rats.Pflugers Arch. 2024 Mar;476(3):337-350. doi: 10.1007/s00424-023-02900-1. Epub 2023 Dec 30. Pflugers Arch. 2024. PMID: 38159130
-
Efficacy of stiripentol in hyperthermia-induced seizures in a mouse model of Dravet syndrome.Epilepsia. 2012 Jul;53(7):1140-5. doi: 10.1111/j.1528-1167.2012.03497.x. Epub 2012 May 11. Epilepsia. 2012. PMID: 22578034
-
Vortioxetine increases absence-like seizures in WAG/Rij rats but decreases penicillin- and pentylenetetrazole-induced seizures in Wistar rats.Epilepsy Behav. 2021 Mar;116:107797. doi: 10.1016/j.yebeh.2021.107797. Epub 2021 Feb 6. Epilepsy Behav. 2021. PMID: 33561766
-
Genetic models of absence epilepsy, with emphasis on the WAG/Rij strain of rats.Epilepsy Res. 1992 Jul;12(2):75-86. doi: 10.1016/0920-1211(92)90029-s. Epilepsy Res. 1992. PMID: 1396543 Review.
Cited by
-
Voltage-gated calcium channels in genetic epilepsies.J Neurochem. 2024 Dec;168(12):3853-3871. doi: 10.1111/jnc.15983. Epub 2023 Oct 11. J Neurochem. 2024. PMID: 37822150 Free PMC article. Review.
-
An Update on Stiripentol Mechanisms of Action: A Narrative Review.Adv Ther. 2024 Apr;41(4):1351-1371. doi: 10.1007/s12325-024-02813-0. Epub 2024 Mar 5. Adv Ther. 2024. PMID: 38443647 Free PMC article. Review.
-
Efficacy of Stiripentol Beyond Dravet Syndrome: A Retrospective Medical Record Review of Patients with Drug-Resistant Epilepsies.Neurol Ther. 2025 Jun;14(3):1129-1150. doi: 10.1007/s40120-025-00755-5. Epub 2025 May 3. Neurol Ther. 2025. PMID: 40319205 Free PMC article.
-
New GABA-Targeting Therapies for the Treatment of Seizures and Epilepsy: II. Treatments in Clinical Development.CNS Drugs. 2023 Sep;37(9):781-795. doi: 10.1007/s40263-023-01025-4. Epub 2023 Aug 21. CNS Drugs. 2023. PMID: 37603261 Free PMC article. Review.
-
Efficacy and tolerability of add-on stiripentol in real-world clinical practice: An observational study in Dravet syndrome and non-Dravet developmental and epileptic encephalopathies.Epilepsia Open. 2024 Feb;9(1):164-175. doi: 10.1002/epi4.12847. Epub 2023 Nov 8. Epilepsia Open. 2024. PMID: 37867433 Free PMC article.
References
-
- Poisson M, Huguet F, Savattier A, Bakri‐Logeais F, Narcisse G. A new type of anticonvulsant, stiripentol. Pharmacological profile and neurochemical study. Arzneimittelforschung. 1984;34:199–204. - PubMed
-
- Quilichini PP, Chiron C, Ben‐Ari Y, Gozlan H. Stiripentol, a putative antiepileptic drug, enhances the duration of opening of GABA‐A receptor channels. Epilepsia. 2006;47:704–16. - PubMed
-
- Sada N, Lee S, Katsu T, Otsuki T, Inoue T. Epilepsy treatment. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science. 2015;347:1362–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

