Anti-KIT monoclonal antibody CDX-0159 induces profound and durable mast cell suppression in a healthy volunteer study
- PMID: 35184297
- PMCID: PMC9544977
- DOI: 10.1111/all.15262
Anti-KIT monoclonal antibody CDX-0159 induces profound and durable mast cell suppression in a healthy volunteer study
Abstract
Background: Mast cells (MC) are powerful inflammatory immune sentinel cells that drive numerous allergic, inflammatory, and pruritic disorders when activated. MC-targeted therapies are approved in several disorders, yet many patients have limited benefit suggesting the need for approaches that more broadly inhibit MC activity. MCs require the KIT receptor and its ligand stem cell factor (SCF) for differentiation, maturation, and survival. Here we describe CDX-0159, an anti-KIT monoclonal antibody that potently suppresses MCs in human healthy volunteers.
Methods: CDX-0159-mediated KIT inhibition was tested in vitro using KIT-expressing immortalized cells and primary human mast cells. CDX-0159 safety and pharmacokinetics were evaluated in a 13-week good laboratory practice (GLP)-compliant cynomolgus macaque study. A single ascending dose (0.3, 1, 3, and 9 mg/kg), double-blinded placebo-controlled phase 1a human healthy volunteer study (n = 32) was conducted to evaluate the safety, pharmacokinetics, and pharmacodynamics of CDX-0159.
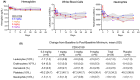
Results: CDX-0159 inhibits SCF-dependent KIT activation in vitro. Fc modifications in CDX-0159 led to elimination of effector function and reduced serum clearance. In cynomolgus macaques, multiple high doses were safely administered without a significant impact on hematology, a potential concern for KIT inhibitors. A single dose of CDX-0159 in healthy human subjects was generally well tolerated and demonstrated long antibody exposure. Importantly, CDX-0159 led to dose-dependent, profound suppression of plasma tryptase, a MC-specific protease associated with tissue MC burden, indicative of systemic MC suppression or ablation.
Conclusion: CDX-0159 administration leads to systemic mast cell ablation and may represent a safe and novel approach to treat mast cell-driven disorders.
Keywords: CDX-0159; KIT; mast cell; monoclonal antibody.
© 2022 Celldex Therapeutics, Inc. Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
DA, MBM, LC, JG, AC, LAV, PAM, LJT, TRH, TK, DY, EC, and MHC are full‐time employees of Celldex Therapeutics. SBS is a full‐time employee of Boehringer‐Ingelheim. MM reports grants and/or personal fees from Allakos, Amgen, Aralez, ArgenX, AstraZeneca, Celldex, Centogene, CSL Behring, FAES, Genentech, GIInnovation, Innate Pharma, Kyowa Kirin, Leo Pharma, Lilly, Menarini, Moxie, Novartis, Roche, Sanofi/Regeneron, Third HarmonicBio, UCB, and Uriach. MK is a full‐time employee of Altasciences, which has received research grant/funding (institution) from Actelion Pharmaceuticals, Acurx Pharmaceuticals, Bioxcel Therapeutics, Grifols, Jazz Pharmaceuticals, Novus Therapeutics, Pfizer, DynPort Vaccine Company, Novo Nordisk, FDA/NIH, and ViroDefense. MK holds a leadership role at Altasciences. JG and RG are inventors in patent applications No: 63/140,642 and 63/140,621.
Figures



References
-
- Varricchi G, Rossi FW, Galdiero MR, et al. Physiological roles of mast cells: collegium internationale allergologicum update 2019. Int Arch Allergy Immunol. 2019;179:247‐261. - PubMed
-
- Dudeck A, Köberle M, Goldmann O, et al. Mast cells as protectors of health. Journal of Allergy and Clinical Immunology. 2019;144:S4‐S18. - PubMed
-
- Dahlin JS, Maurer M, Metcalfe DD, Pejler G, Sagi‐Eisenberg R, Nilsson G. The ingenious mast cell: contemporary insights into mast cell behavior and function. Allergy. 2021;77(1):83‐99. - PubMed
-
- Maurer M, Köberle M, Metz M, Biedermann T. Mast cells: Promoters of health and modulators of disease. Journal of Allergy and Clinical Immunology. 2019;144:S1‐S3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

