Effects of a comprehensive medication review intervention on health-related quality of life and other clinical outcomes in geriatric outpatients with polypharmacy: A pragmatic randomized clinical trial
- PMID: 35184324
- PMCID: PMC9314627
- DOI: 10.1111/bcp.15287
Effects of a comprehensive medication review intervention on health-related quality of life and other clinical outcomes in geriatric outpatients with polypharmacy: A pragmatic randomized clinical trial
Abstract
Aim: To investigate the effects of a comprehensive medication review intervention on health-related quality of life (HRQoL) and clinical outcomes in geriatric outpatients exposed to polypharmacy.
Methods: Pragmatic, nonblinded, randomized clinical trial with follow-up after 4 and 13 months. Participants were geriatric outpatients taking ≥9 medicines. The intervention was an additional consultation with a physician focusing on reviewing medication, informing patients about their medicines and increasing cross-sectoral communication as supplement to and compared with usual care. The primary outcome was change in HRQoL after 4 months measured with the EuroQoL 5-dimension 5-level (EQ-5D-5L) questionnaire. Secondary outcomes were HRQoL after 13 months, mortality, admissions, falls and number of medicines after 4 and 13 months.
Results: Of 785 eligible patients, 408 were included (age: mean 80.6 [standard deviation 7.22] years; number of medicines: median 12 [interquartile range 10-14]; females 71%). After 4 months, the adjusted between-group difference in EQ-5D-5L index score was 0.066 in favour of the medication consultation (95% confidence interval 0.01 to 0.12, P = .02). After 4 months, two (1%) participants had died in the medication-consultation group and nine (4%) in the usual-care group (log-rank test, P = .045). The medication consultation reduced the number of medicines by 2.0 (15.8%) after 4 months and 1.3 (10.7%) after 13 months. There were no statistically significant differences in mortality or HRQoL after 13 months, and no differences in falls or admissions.
Conclusions: An additional consultation with medication review and increased communication as supplement to usual geriatric outpatient care improved HRQoL and reduced mortality after 4 months.
Keywords: geriatrics; health-related quality of life; medication reviews; polypharmacy.
© 2022 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
No competing interests have been declared by the authors.
Figures
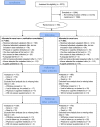
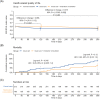
References
-
- Kornholt J, Christensen MB. Prevalence of polypharmacy in Denmark. Dan Med J. 2020;67:A12190680. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

