The central role of Sphingosine kinase 1 in the development of neuroendocrine prostate cancer (NEPC): A new targeted therapy of NEPC
- PMID: 35184376
- PMCID: PMC8858611
- DOI: 10.1002/ctm2.695
The central role of Sphingosine kinase 1 in the development of neuroendocrine prostate cancer (NEPC): A new targeted therapy of NEPC
Abstract
Background: Neuroendocrine prostate cancer (NEPC) is often diagnosed as a sub-type from the castration-resistant prostate cancer (CRPC) recurred from the second generation of anti-androgen treatment and is a rapidly progressive fatal disease. The molecular mechanisms underlying the trans-differentiation from CRPC to NEPC are not fully characterized, which hampers the development of effective targeted therapy.
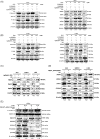
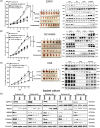
Methods: Bioinformatic analyses were conducted to determine the clinical correlation of sphingosine kinase 1 (SphK1) in CRPC progression. To investigate the transcriptional regulation SphK1 and neuroendocrine (NE) transcription factor genes, both chromosome immunoprecipitation and luciferase reporter gene assays were performed. To demonstrate the role of SphK1 in NEPC development, neurosphere assay was carried out along with several biomarkers determined by quantitative PCR and western blot. Furthermore, in vivo NEPC xenograft models and patient-derived xenograft (PDX) model were employed to determine the effect of SphK1 inhibitors and target validation.
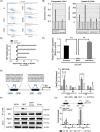
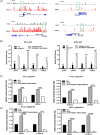
Results: Significant prevalence of SphK1 in NEPC development is observed from clinical datasets. SphK1 is transcriptionally repressed by androgen receptor-RE1-silencing transcription factor (REST) complex. Furthermore, sphingosine 1-phosphate produced by SphK1 can modulate REST protein turnover via MAPK signaling pathway. Also, decreased REST protein levels enhance the expression of NE markers in CRPC, enabling the transition to NEPC. Finally, specific SphK1 inhibitors can effectively inhibit the growth of NEPC tumors and block the REST protein degradation in PDX.
Conclusions: SphK1 plays a central role in NEPC development, which offers a new target for this lethal cancer using clinically approved SphK1 inhibitors.
Keywords: Sphingosine kinase 1; neuroendocrine prostate cancer; targeted therapy; therapy and castration resistant prostate cancer.
© 2022 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Virgo KS, Basch E, Loblaw DA, et al. Second‐line hormonal therapy for men with chemotherapy‐naive, castration‐resistant prostate cancer: American Society of Clinical Oncology Provisional Clinical Opinion. J Clin Oncol. 2017;35(17):1952‐1964. - PubMed
-
- Ritch CR, Cookson MS. Advances in the management of castration resistant prostate cancer. BMJ. 2016;355:i4405. - PubMed
-
- Conteduca V, Aieta M, Amadori D, De Giorgi U. Neuroendocrine differentiation in prostate cancer: current and emerging therapy strategies. Crit Rev Oncol Hematol. 2014;92(1):11‐24. - PubMed
-
- Santoni M, Conti A, Burattini L, et al. Neuroendocrine differentiation in prostate cancer: novel morphological insights and future therapeutic perspectives. Biochim Biophys Acta. 2014;1846(2):630‐637. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Prostate Cancer Foundation, USA
- MOST 108-2911-I-005-509/The Ministry of Science and Technology in Taiwan
- MOST 110-2926-I-005-502/The Ministry of Science and Technology in Taiwan
- MOST 104-2911-I-002-578/The Ministry of Science and Technology in Taiwan
- MOST 105-2911-I-002-521/The Ministry of Science and Technology in Taiwan
LinkOut - more resources
Full Text Sources
Medical
