The dynamics of cell death patterns and regeneration during acute liver injury in mice
- PMID: 35184410
- PMCID: PMC9063440
- DOI: 10.1002/2211-5463.13383
The dynamics of cell death patterns and regeneration during acute liver injury in mice
Abstract
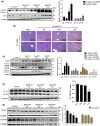
Acute liver injury is a serious clinical syndrome with multiple causes and unclear pathological process. Here, CCl4 - and D-galactosamine/lipopolysaccharide (D-gal/LPS)-induced acute liver injury was established to explore the cell death patterns and determine whether or not liver regeneration occurred. In CCl4 -induced hepatic injury, three phases, including the early, progressive, and recovery phase, were considered based on alterations of serum transaminases and liver morphology. Moreover, in this model, cytokines exhibited double-peak fluctuations; apoptosis and pyroptosis persisted throughout all phases; autophagy occurred in the early and the progressive phases; and sufficient and timely hepatocyte regeneration was observed only during the recovery phase. All of these phenomena contribute to mild liver injury and subsequent regeneration. Strikingly, only the early and progressive phases were observed in the D-gal/LPS model. Slight pyroptosis occurred in the early phase but diminished in the progressive phase, while apoptosis, reduced autophagy, and slight but subsequently diminished regeneration occurred only during the progressive phase, accompanied by a strong cytokine storm, resulting in severe liver injury with high mortality. Taken together, our work reveals variable modes and dynamics of cell death and regeneration, which lead to different consequences for mild and severe acute liver injury, providing a helpful reference for clinical therapy and prognosis.
Keywords: acute liver injury; cell death; cytokines; hepatocyte; prognosis; regeneration.
© 2022 The Authors. FEBS Open Bio published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Stravitz RT, Lee WM. Acute liver failure. Lancet (London, England). 2019;394:869–81.
-
- Iorga A, Dara L. Cell death in drug‐induced liver injury. Adv Pharmacol (San Diego, Calif). 2019;85:31–74.
-
- Zhao Y, Shi J, Shao F. Inflammatory caspases: activation and cleavage of gasdermin‐D in vitro and during pyroptosis. Methods Mol Biol (Clifton, NJ). 2018;1714:131–48.
-
- Guo H, Xie M, Zhou C, Zheng M. The relevance of pyroptosis in the pathogenesis of liver diseases. Life Sci. 2019;223:69–73. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

