Prodromal Parkinson's disease: hype or hope for disease-modification trials?
- PMID: 35184752
- PMCID: PMC8859908
- DOI: 10.1186/s40035-022-00286-1
Prodromal Parkinson's disease: hype or hope for disease-modification trials?
Abstract
The ultimate goal in Parkinson's disease (PD) research remains the identification of treatments that are capable of slowing or even halting the progression of the disease. The failure of numerous past disease-modification trials in PD has been attributed to a variety of factors related not only to choosing wrong interventions, but also to using inadequate trial designs and target populations. In patients with clinically established PD, neuronal pathology may already have advanced too far to be modified by any intervention. Based on such reasoning, individuals in yet prediagnostic or prodromal disease stages, may provide a window of opportunity to test disease-modifying strategies. There is now sufficient evidence from prospective studies to define diagnostic criteria for prodromal PD and several approaches have been studied in observational cohorts. These include the use of PD-risk algorithms derived from multiple established risk factors for disease as well as follow-up of cohorts with single defined prodromal markers like hyposmia, rapid eye movement sleep behavior disorders, or PD gene carriers. In this review, we discuss recruitment strategies for disease-modification trials in various prodromal PD cohorts, as well as potential trial designs, required trial durations, and estimated sample sizes. We offer a concluding outlook on how the goal of implementing disease-modification trials in prodromal cohorts might be achieved in the future.
Keywords: Epidemiology; Neuroprotection; Neuroprotective; Preclinical; Prevention; Preventive; Probability; Randomized controlled trial.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
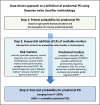
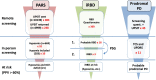
References
-
- Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30:1591–1601. - PubMed
-
- Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, et al. Parkinson disease. Nat Rev Dis Prim. 2017;3:1–21. - PubMed
-
- Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114:2283–2301. - PubMed
-
- Greffard S, Verny M, Bonnet AM, Beinis JY, Gallinari C, Meaume S, et al. Motor score of the unified Parkinson disease rating scale as a good predictor of Lewy body-associated neuronal loss in the substantia nigra. Arch Neurol. 2006;63:584–588. - PubMed
-
- Del Tredici K, Braak H. Lewy pathology and neurodegeneration in premotor Parkinson’s disease. Mov Disord. 2012;27:597–607. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

