Effect of Shengbai Decoction on Chemotherapy-Induced Myelosuppression and Survival of Gastric Cancer Patients After Radical Resection: A Retrospective Study
- PMID: 35185148
- PMCID: PMC8876002
- DOI: 10.12659/MSM.935936
Effect of Shengbai Decoction on Chemotherapy-Induced Myelosuppression and Survival of Gastric Cancer Patients After Radical Resection: A Retrospective Study
Abstract
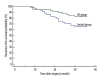
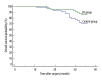
BACKGROUND Myelosuppression is one of the most common chemotherapy-induced adverse events and results in a series of clinical symptoms. This study aimed to evaluate the effect of Shengbai decoction (SD) on chemotherapy-induced myelosuppression and survival of gastric cancer (GC) patients after radical resection. MATERIAL AND METHODS We retrospectively analyzed data from 115 patients with stage II-III GC who underwent adjuvant chemotherapy after radical resection between May 2015 and June 2017 in our hospital. Among these patients, 57 received Shengbai decoction along with adjuvant chemotherapy (SD group), while 58 received adjuvant chemotherapy alone (control group). Medical records, including adverse events, the treatment completion rate of adjuvant chemotherapy, 3-year overall survival (OS), and 3-year recurrence-free survival (RFS), were compared. RESULTS Patient characteristics did not differ significantly between the 2 groups. No adverse events related to Shengbai decoction were reported in the SD group. Patients in the SD group had less neutropenia (P=0.0430), thrombocytopenia (P=0.0323), and anemia (P=0.0497). The SD group had a significantly lower probability of dose reduction (P=0.0448). The completion rate of adjuvant chemotherapy of the SD group was considerably higher than that of the control group (P=0.0398). The SD group had a significantly better 3-year RFS (P=0.0369) and 3-year OS (P=0.0455) than the control group. CONCLUSIONS Shengbai decoction effectively improved postoperative survival of patients with GC by alleviating chemotherapy-induced myelosuppression and improving the completion rate of adjuvant chemotherapy.
Conflict of interest statement
Figures
Similar articles
-
[Safety and efficacy of adjuvant chemotherapy with oxaliplatin and S-1 for patients with locally advanced gastric cancer after D2 lymph nodes dissection].Zhonghua Wei Chang Wai Ke Za Zhi. 2021 Feb 25;24(2):145-152. doi: 10.3760/cma.j.cn.441530-20201016-00561. Zhonghua Wei Chang Wai Ke Za Zhi. 2021. PMID: 33508920 Chinese.
-
Adjuvant albumin-bound paclitaxel combined with S-1 vs. oxaliplatin combined with capecitabine after D2 gastrectomy in patients with stage III gastric adenocarcinoma: a phase III multicenter, open-label, randomized controlled clinical trial protocol.BMC Cancer. 2021 Jan 12;21(1):56. doi: 10.1186/s12885-020-07772-7. BMC Cancer. 2021. PMID: 33435909 Free PMC article.
-
Effect of Yiqi Huayu Jiedu decoction on stages II and III gastric cancer: A multicenter, prospective, cohort study.Medicine (Baltimore). 2019 Nov;98(47):e17875. doi: 10.1097/MD.0000000000017875. Medicine (Baltimore). 2019. PMID: 31764782 Free PMC article.
-
[The value of adjuvant and neoadjuvant chemotherapy in treatment of stomach carcinoma].Wien Klin Wochenschr. 1996;108(16):496-504. Wien Klin Wochenschr. 1996. PMID: 8967093 Review. German.
-
Comparison of S-1-based vs. capecitabine-based adjuvant chemotherapy for patients with gastric cancer: a systematic review and meta-analysis.Eur J Clin Pharmacol. 2021 Dec;77(12):1791-1804. doi: 10.1007/s00228-021-03187-w. Epub 2021 Jul 17. Eur J Clin Pharmacol. 2021. PMID: 34275019
References
-
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- Sasako M, Inoue M, Lin JT, et al. Gastric Cancer Working Group report. Jpn J Clin Oncol. 2010;40(Suppl 1):i28–37. - PubMed
-
- Coburn N, Cosby R, Klein L, et al. Staging and surgical approaches in gastric cancer: A systematic review. Cancer Treat Rev. 2018;63:104–15. - PubMed
-
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11(5):439–49. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

