Activation of RAS/MAPK pathway confers MCL-1 mediated acquired resistance to BCL-2 inhibitor venetoclax in acute myeloid leukemia
- PMID: 35185150
- PMCID: PMC8858957
- DOI: 10.1038/s41392-021-00870-3
Activation of RAS/MAPK pathway confers MCL-1 mediated acquired resistance to BCL-2 inhibitor venetoclax in acute myeloid leukemia
Erratum in
-
Correction: Activation of RAS/MAPK pathway confers MCL-1 mediated acquired resistance to BCL-2 inhibitor venetoclax in acute myeloid leukemia.Signal Transduct Target Ther. 2022 Apr 1;7(1):110. doi: 10.1038/s41392-022-00958-4. Signal Transduct Target Ther. 2022. PMID: 35365596 Free PMC article. No abstract available.
Abstract
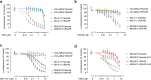
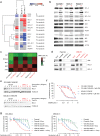
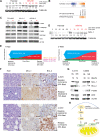
Despite high initial response rates, acute myeloid leukemia (AML) treated with the BCL-2-selective inhibitor venetoclax (VEN) alone or in combinations commonly acquires resistance. We performed gene/protein expression, metabolomic and methylation analyses of isogenic AML cell lines sensitive or resistant to VEN, and identified the activation of RAS/MAPK pathway, leading to increased stability and higher levels of MCL-1 protein, as a major acquired mechanism of VEN resistance. MCL-1 sustained survival and maintained mitochondrial respiration in VEN-RE cells, which had impaired electron transport chain (ETC) complex II activity, and MCL-1 silencing or pharmacologic inhibition restored VEN sensitivity. In support of the importance of RAS/MAPK activation, we found by single-cell DNA sequencing rapid clonal selection of RAS-mutated clones in AML patients treated with VEN-containing regimens. In summary, these findings establish RAS/MAPK/MCL-1 and mitochondrial fitness as key survival mechanisms of VEN-RE AML and provide the rationale for combinatorial strategies effectively targeting these pathways.
© 2022. The Author(s).
Conflict of interest statement
R.A.P. received financial support from AbbVie and Genentech. A.D.S. has received honorarium from Takeda, Novartis, Jazz, and Otsuka Pharmaceuticals and research support from Medivir AB and Takeda. A.D.S. is named as an inventor on a patent application related to the use of DNT cells in AML. A.D.S. owns stock in AbbVie Pharmaceuticals. A.D.S holds the Ronald N. Buick Chair in Oncology Research. MK: Consulting/honorarium: AbbVie, Genentech, F. Hoffman La-Roche, Stemline Therapeutics, Amgen, Forty-Seven, Kisoji; research funding/clinical trials support: AbbVie, Genentech, F. Hoffman La-Roche, Eli Lilly, Cellectis, Calithera, Ablynx, Stemline Therapeutics, Agios, Ascentage, Astra Zeneca, Forty-Seven, Kisoji; stock options/royalties: Reata Pharmaceutical.
Figures


References
-
- DiNardo CD, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19:216–228. - PubMed
-
- Maiti A, et al. Outcomes in Molecular Subgroups and Resistance Patterns with Ten-Day Decitabine and Venetoclax (DEC10-VEN) in Acute Myeloid Leukemia. Blood. 2019;134:645–645.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

