Soluble Urokinase Receptor and Mortality in Kidney Transplant Recipients
- PMID: 35185364
- PMCID: PMC8842271
- DOI: 10.3389/ti.2021.10071
Soluble Urokinase Receptor and Mortality in Kidney Transplant Recipients
Abstract
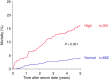
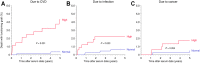
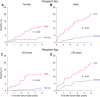
Main problem: Soluble urokinase plasminogen activator receptor (suPAR) is an immunological risk factor for kidney disease and a prognostic marker for cardiovascular events. Methods: We measured serum suPAR levels in a total of 1,023 kidney transplant recipients either before (cohort 1, n = 474) or at year 1 after transplantation (cohort 2, n = 549). The association of suPAR levels and all-cause and cardiovascular mortality was evaluated by multivariable Cox regression analysis. Results: The highest suPAR tertile compared to the two lower tertiles had a significantly higher risk of all-cause mortality in both cohorts separately (cohort 1: hazard ratio (HR) 1.92, 95% confidence interval (CI) 1.20-3.08, p = 0.007; cohort 2: HR = 2.78, 95% CI 1.51-5.13, p = 0.001) and combined (n = 1,023, combined HR = 2.14, 95% CI 1.48-3.08, p < 0.001). The association remained significant in the subgroup of patients with normal kidney function (cohort 2: HR = 5.40, 95% CI 1.42-20.5, p = 0.013). The increased mortality risk in patients with high suPAR levels was attributable mainly to an increased rate of cardiovascular death (n = 1,023, HR = 4.24, 95% CI 1.81-9.96, p < 0.001). Conclusion: A high suPAR level prior to and at 1 year after kidney transplantation was associated with an increased risk of patient death independent of kidney function, predominantly from cardiovascular cause.
Keywords: cardiovascular; kidney; mortality; suPAR; transplantation.
Copyright © 2022 Morath, Hayek, Döhler, Nusshag, Sommerer, Zeier, Reiser and Süsal.
Conflict of interest statement
CM and MZ, together with the University of Heidelberg, are co-founders of TolerogenixX GmbH, Heidelberg, Germany, a biotechnology company that holds licenses for cell therapies. CM, CSü, and MZ filed a patent application for a cell therapy. JR is cofounder of Trisaq, a biotechnology company developing drugs targeting suPAR. SH and JR are members of the scientific advisory board of Trisaq. JR holds patents and licenses related to suPAR. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

