Bio-Engineering of Pre-Vascularized Islet Organoids for the Treatment of Type 1 Diabetes
- PMID: 35185372
- PMCID: PMC8842259
- DOI: 10.3389/ti.2021.10214
Bio-Engineering of Pre-Vascularized Islet Organoids for the Treatment of Type 1 Diabetes
Abstract
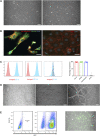
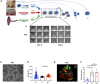
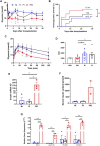
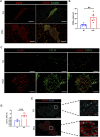
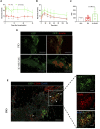
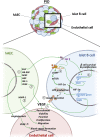
Lack of rapid revascularization and inflammatory attacks at the site of transplantation contribute to impaired islet engraftment and suboptimal metabolic control after clinical islet transplantation. In order to overcome these limitations and enhance engraftment and revascularization, we have generated and transplanted pre-vascularized insulin-secreting organoids composed of rat islet cells, human amniotic epithelial cells (hAECs), and human umbilical vein endothelial cells (HUVECs). Our study demonstrates that pre-vascularized islet organoids exhibit enhanced in vitro function compared to native islets, and, most importantly, better engraftment and improved vascularization in vivo in a murine model. This is mainly due to cross-talk between hAECs, HUVECs and islet cells, mediated by the upregulation of genes promoting angiogenesis (vegf-a) and β cell function (glp-1r, pdx1). The possibility of adding a selected source of endothelial cells for the neo-vascularization of insulin-scereting grafts may also allow implementation of β cell replacement therapies in more favourable transplantation sites than the liver.
Keywords: HUVECs; human amniotic epithelial cells; prevascularized iset organoids; regenerative medicine; tissue engineering; β cell replacement therapies.
Copyright © 2022 Wassmer, Lebreton, Bellofatto, Perez, Cottet-Dumoulin, Andres, Bosco, Berney, Othenin-Girard, Martinez De Tejada, Cohen, Olgasi, Follenzi, Berishvili and the VANGUARD Consortium.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Insulin-producing organoids engineered from islet and amniotic epithelial cells to treat diabetes.Nat Commun. 2019 Oct 3;10(1):4491. doi: 10.1038/s41467-019-12472-3. Nat Commun. 2019. PMID: 31582751 Free PMC article.
-
Generation of Insulin-Producing Multicellular Organoids.Methods Mol Biol. 2023;2592:37-60. doi: 10.1007/978-1-0716-2807-2_3. Methods Mol Biol. 2023. PMID: 36507984
-
Revolutionizing pancreatic islet organoid transplants: Improving engraftment and exploring future frontiers.Life Sci. 2024 Apr 15;343:122545. doi: 10.1016/j.lfs.2024.122545. Epub 2024 Mar 6. Life Sci. 2024. PMID: 38458556 Review.
-
Improvement of islet transplantation by the fusion of islet cells with functional blood vessels.EMBO Mol Med. 2021 Jan 11;13(1):e12616. doi: 10.15252/emmm.202012616. Epub 2020 Nov 2. EMBO Mol Med. 2021. PMID: 33135383 Free PMC article.
-
Bioengineering and vascularization strategies for islet organoids: advancing toward diabetes therapy.Metabolism. 2024 Mar;152:155786. doi: 10.1016/j.metabol.2024.155786. Epub 2024 Jan 10. Metabolism. 2024. PMID: 38211697 Review.
Cited by
-
The role of endothelial cells in pancreatic islet development, transplantation and culture.Front Cell Dev Biol. 2025 Apr 22;13:1558137. doi: 10.3389/fcell.2025.1558137. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40330424 Free PMC article. Review.
-
Studies on Treatment Within the Scope of Medical Biotechnology for Pancreatic Diseases.Mol Biotechnol. 2025 Apr;67(4):1321-1335. doi: 10.1007/s12033-024-01142-5. Epub 2024 Apr 16. Mol Biotechnol. 2025. PMID: 38627328 Review.
-
Scaffold-free endocrine tissue engineering: role of islet organization and implications in type 1 diabetes.BMC Endocr Disord. 2025 Apr 21;25(1):107. doi: 10.1186/s12902-025-01919-y. BMC Endocr Disord. 2025. PMID: 40259265 Free PMC article. Review.
-
Application and challenge of pancreatic organoids in therapeutic research.Front Pharmacol. 2024 May 24;15:1366417. doi: 10.3389/fphar.2024.1366417. eCollection 2024. Front Pharmacol. 2024. PMID: 38855754 Free PMC article. Review.
-
Legal and Regulatory Challenges for Emerging Regenerative Medicine Solutions for Diabetes.Transplantation. 2024 May 1;108(5):1072-1079. doi: 10.1097/TP.0000000000004797. Epub 2023 Sep 26. Transplantation. 2024. PMID: 37749797 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

