Long-Term Sonographical Follow-Up of Arterial Stenosis Due to Spontaneous Cervical Artery Dissection
- PMID: 35185752
- PMCID: PMC8850833
- DOI: 10.3389/fneur.2021.792321
Long-Term Sonographical Follow-Up of Arterial Stenosis Due to Spontaneous Cervical Artery Dissection
Abstract
Purpose: Little is known about the long-term course of arterial stenosis after spontaneous cervical artery dissection (sCAD). We analyzed changes over time and evaluated factors potentially associated with these changes and recurring sCAD.
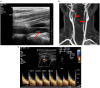
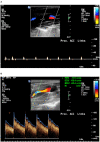
Materials and methods: Adult patients with sCAD, admitted to our neurological department between 2004 and 2018, were included. All patients underwent initial and follow-up repetitive neurovascular ultrasound for a mean duration of 15.3 ± 21 months. Clinical and imaging data were registered for each patient.
Results: A total of 259 sCADs were diagnosed in 224 patients. Either internal carotid arteries (n = 133, 59.4%), vertebral arteries (n = 58, 25.9%), or multiple arteries (n = 33, 14.7%) were affected. In 93 out of 183 patients (51%), and in 117 out of 210 arteries under investigation (55.7%), vascular stenosis decreased over time. Occluded arteries recanalized early in 34 (54%) and stayed occluded in 29 patients (46.0%). Of 145 initially hemodynamically relevant stenosis, 77 (53.1%) improved over time. Overall, 12 patients (5.4 %) had a recurring sCAD during follow-up. Pseudoaneurysms were found in 19 patients.
Conclusion: The sonographical course of sCAD is highly dynamic within the first year after disease onset and should be monitored carefully. Decreasing degrees of stenosis and recanalization of occluded arteries occurred in half of all patients. Recurrent sCAD was a rare event in our cohort.
Keywords: neurovascular ultrasound; rare causes of stroke; spontaneous cervical artery dissection; stenosis; stroke.
Copyright © 2022 Strunk, Schwindt, Wiendl, Dittrich and Minnerup.
Conflict of interest statement
HW is a member of the following scientific advisory boards/steering committees: Biogen, Sanofi Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, and Roche. HW has received speaker honoraria and travel support from Alexion, Biogen, Cognomed, Evgen, Sanofi Genzyme, Impulze, KWHC, Merck Serono, Novartis, PeerVoice, Pennside, and PSL Group. HW has received compensation as a consultant from AbbVie, Actelion, Biogen, Sanofi Genzyme, Novartis, and Roche. HW has received research support from Biogen, Sanofi Genzyme, GlaxoSmithKline, Roche, and Solace Pharmaceuticals UK. JM has received grants from Deutsche Forschungsgemeinschaft, Bundesministerium für Bildung und Forschung BMBF, Else Kröner-Fresenius-Stiftung, EVER Pharma Jena GmbH, and Ferrer International, travel grants from Boehringer Ingelheim, and speaking fees from Bayer Vital. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources

