Are Bacterial Persisters Dormant Cells Only?
- PMID: 35185807
- PMCID: PMC8847742
- DOI: 10.3389/fmicb.2021.708580
Are Bacterial Persisters Dormant Cells Only?
Abstract
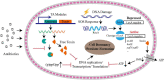
Bacterial persisters are a sub-population of phenotypic variants that tolerate high concentrations of antibiotics within the genetically homogeneous cells. They resume division upon the removal of drugs. Bacterial persistence is one of major causes of antibiotic treatment failure and recurrent infection. Cell dormancy, triggered by toxin/antitoxin pair, (p)ppGpp, SOS response and ATP levels, is known to be the mechanistic basis for persistence. However, recent studies have demonstrated that bacteria with active metabolism can maintain persistence by lowering intracellular antibiotic concentration via an efflux pump. Additionally, others and our work have showed that cell wall deficient bacteria (CWDB), including both L-form and spheroplasts that produced by β-lactam antibiotics, are associated with antibiotic persistence. They are not dormant cells as their cell walls have been completely damaged. In this review, we discuss the various types of persisters and highlight the contribution of non-walled bacteria on bacterial persistence.
Keywords: L-form; cell wall deficient bacteria; dormancy; persister; spheroplast.
Copyright © 2022 Zou, Peng, Qu and Zheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Active efflux in dormant bacterial cells - New insights into antibiotic persistence.Drug Resist Updat. 2017 Jan;30:7-14. doi: 10.1016/j.drup.2016.11.002. Epub 2016 Nov 29. Drug Resist Updat. 2017. PMID: 28363336 Review.
-
Non-walled spherical Acinetobacter baumannii is an important type of persister upon β-lactam antibiotic treatment.Emerg Microbes Infect. 2020 Dec;9(1):1149-1159. doi: 10.1080/22221751.2020.1770630. Emerg Microbes Infect. 2020. PMID: 32419626 Free PMC article.
-
Prophages and Growth Dynamics Confound Experimental Results with Antibiotic-Tolerant Persister Cells.mBio. 2017 Dec 12;8(6):e01964-17. doi: 10.1128/mBio.01964-17. mBio. 2017. PMID: 29233898 Free PMC article.
-
Host Cell Oxidative Stress Induces Dormant Staphylococcus aureus Persisters.Microbiol Spectr. 2022 Feb 23;10(1):e0231321. doi: 10.1128/spectrum.02313-21. Epub 2022 Feb 23. Microbiol Spectr. 2022. PMID: 35196815 Free PMC article.
-
Plausible role of bacterial toxin-antitoxin system in persister cell formation and elimination.Mol Oral Microbiol. 2019 Jun;34(3):97-107. doi: 10.1111/omi.12258. Epub 2019 May 2. Mol Oral Microbiol. 2019. PMID: 30891951 Review.
Cited by
-
Bacterial Persister Cells and Development of Antibiotic Resistance in Chronic Infections: An Update.Br J Biomed Sci. 2024 Aug 7;81:12958. doi: 10.3389/bjbs.2024.12958. eCollection 2024. Br J Biomed Sci. 2024. PMID: 39170669 Free PMC article. Review.
-
Cancer drug-tolerant persister cells: from biological questions to clinical opportunities.Nat Rev Cancer. 2024 Oct;24(10):694-717. doi: 10.1038/s41568-024-00737-z. Epub 2024 Sep 2. Nat Rev Cancer. 2024. PMID: 39223250 Review.
-
The artemisinin-induced dormant stages of Plasmodium falciparum exhibit hallmarks of cellular quiescence/senescence and drug resilience.Nat Commun. 2024 Aug 29;15(1):7485. doi: 10.1038/s41467-024-51846-0. Nat Commun. 2024. PMID: 39209862 Free PMC article.
-
A bacterial toxin-antitoxin system involved in an unusual response to genotoxic stress.EMBO Rep. 2025 Aug 18. doi: 10.1038/s44319-025-00545-y. Online ahead of print. EMBO Rep. 2025. PMID: 40825874
-
Non-Canonical Aspects of Antibiotics and Antibiotic Resistance.Antibiotics (Basel). 2024 Jun 17;13(6):565. doi: 10.3390/antibiotics13060565. Antibiotics (Basel). 2024. PMID: 38927231 Free PMC article. Review.
References
-
- Alexander-Jackson E. (1945). A hitherto undemonstrated zoogleal form of mycobacterium tuberculosis. Ann. N. Y. Acad. Sci. 46 127–152. 10.1111/j.1749-6632.1945.tb36166.x - DOI
Publication types
LinkOut - more resources
Full Text Sources

