Staphylococcus aureus β-Toxin Exerts Anti-angiogenic Effects by Inhibiting Re-endothelialization and Neovessel Formation
- PMID: 35185854
- PMCID: PMC8851161
- DOI: 10.3389/fmicb.2022.840236
Staphylococcus aureus β-Toxin Exerts Anti-angiogenic Effects by Inhibiting Re-endothelialization and Neovessel Formation
Abstract
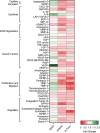
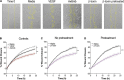
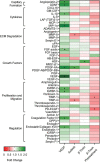
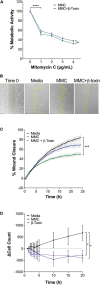
Staphylococcus aureus causes severe, life-threatening infections that often are complicated by severe local and systemic pathologies with non-healing lesions. A classic example is S. aureus infective endocarditis (IE), where the secreted hemolysin β-toxin potentiates the disease via its sphingomyelinase and biofilm ligase activities. Although these activities dysregulate human aortic endothelial cell activation, β-toxin effect on endothelial cell function in wound healing has not been addressed. With the use of the ex vivo rabbit aortic ring model, we provide evidence that β-toxin prevents branching microvessel formation, highlighting its ability to interfere with tissue re-vascularization and vascular repair. We show that β-toxin specifically targets both human aortic endothelial cell proliferation and cell migration and inhibits human umbilical vein endothelial cell rearrangement into capillary-like networks in vitro. Proteome arrays specific for angiogenesis-related molecules provided evidence that β-toxin promotes an inhibitory profile in endothelial cell monolayers, specifically targeting production of TIMP-1, TIMP-4, and IGFBP-3 to counter the effect of a pro-angiogenic environment. Dysregulation in the production of these molecules is known to result in sprouting defects (including deficient cell proliferation, migration, and survival), vessel instability and/or vascular regression. When endothelial cells are grown under re-endothelialization/wound healing conditions, β-toxin decreases the pro-angiogenic molecule MMP-8 and increases the anti-angiogenic molecule endostatin. Altogether, the data indicate that β-toxin is an anti-angiogenic virulence factor and highlight a mechanism where β-toxin exacerbates S. aureus invasive infections by interfering with tissue re-vascularization and vascular repair.
Keywords: Staphylococcus aureus; angiogenesis; endothelial cell; sphingomyelinase (SMase); β-toxin.
Copyright © 2022 Tran, Tang and Salgado-Pabón.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Staphylococcal β-Toxin Modulates Human Aortic Endothelial Cell and Platelet Function through Sphingomyelinase and Biofilm Ligase Activities.mBio. 2017 Mar 21;8(2):e00273-17. doi: 10.1128/mBio.00273-17. mBio. 2017. PMID: 28325766 Free PMC article.
-
Staphylococcus aureus β-Toxin Mutants Are Defective in Biofilm Ligase and Sphingomyelinase Activity, and Causation of Infective Endocarditis and Sepsis.Biochemistry. 2016 May 3;55(17):2510-7. doi: 10.1021/acs.biochem.6b00083. Epub 2016 Apr 15. Biochemistry. 2016. PMID: 27015018 Free PMC article.
-
ϕSa3mw Prophage as a Molecular Regulatory Switch of Staphylococcus aureus β-Toxin Production.J Bacteriol. 2019 Jun 21;201(14):e00766-18. doi: 10.1128/JB.00766-18. Print 2019 Jul 15. J Bacteriol. 2019. PMID: 30962356 Free PMC article.
-
Staphylococcus aureus β-toxin production is common in strains with the β-toxin gene inactivated by bacteriophage.J Infect Dis. 2014 Sep 1;210(5):784-92. doi: 10.1093/infdis/jiu146. Epub 2014 Mar 11. J Infect Dis. 2014. PMID: 24620023 Free PMC article.
-
Hydrogen sulphide and angiogenesis: mechanisms and applications.Br J Pharmacol. 2011 Oct;164(3):853-65. doi: 10.1111/j.1476-5381.2010.01191.x. Br J Pharmacol. 2011. PMID: 21198548 Free PMC article. Review.
Cited by
-
Potential Compounds as Inhibitors of Staphylococcal Virulence Factors Involved in the Development of Thrombosis.Toxins (Basel). 2025 Jul 4;17(7):340. doi: 10.3390/toxins17070340. Toxins (Basel). 2025. PMID: 40711151 Free PMC article. Review.
-
In Silico Genome-Scale Analysis of Molecular Mechanisms Contributing to the Development of a Persistent Infection with Methicillin-Resistant Staphylococcus aureus (MRSA) ST239.Int J Mol Sci. 2022 Dec 16;23(24):16086. doi: 10.3390/ijms232416086. Int J Mol Sci. 2022. PMID: 36555727 Free PMC article.
-
SEC is an antiangiogenic virulence factor that promotes Staphylococcus aureus endocarditis independent of superantigen activity.Sci Adv. 2022 May 13;8(19):eabo1072. doi: 10.1126/sciadv.abo1072. Epub 2022 May 11. Sci Adv. 2022. PMID: 35544579 Free PMC article.
-
Vegetation Formation in Staphylococcus Aureus Endocarditis Inversely Correlates With RNAIII and sarA Expression in Invasive Clonal Complex 5 Isolates.Front Cell Infect Microbiol. 2022 Jul 4;12:925914. doi: 10.3389/fcimb.2022.925914. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35860377 Free PMC article.
-
Augmenting the Angiogenic Profile and Functionality of Cord Blood Endothelial Colony-Forming Cells by Indirect Priming with Bone-Marrow-Derived Mesenchymal Stromal Cells.Biomedicines. 2023 May 5;11(5):1372. doi: 10.3390/biomedicines11051372. Biomedicines. 2023. PMID: 37239042 Free PMC article.
References
-
- Adair T. H., Montani J.-P. (2010). “Colloquium series on integrated systems physiology: from molecule to function to disease,” in Angiogenesis, eds Granger D. N., Granger J. P. (San Rafael, CA: Morgan & Claypool Life Sciences; ). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

