Neutrophils Contribute to ER Stress in Lung Epithelial Cells in the Pristane-Induced Diffuse Alveolar Hemorrhage Mouse Model
- PMID: 35185885
- PMCID: PMC8850275
- DOI: 10.3389/fimmu.2022.790043
Neutrophils Contribute to ER Stress in Lung Epithelial Cells in the Pristane-Induced Diffuse Alveolar Hemorrhage Mouse Model
Abstract
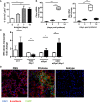
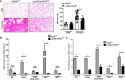
Diffuse alveolar hemorrhage (DAH), although rare, is a life-threatening complication of systemic lupus erythematosus (SLE). Little is known about the pathophysiology of DAH in humans, although increasingly neutrophils, NETosis and inflammatory monocytes have been shown to play an important role in the pristane-induced model of SLE which develops lung hemorrhage and recapitulates many of the pathologic features of human DAH. Using this experimental model, we asked whether endoplasmic reticulum (ER) stress played a role in driving the pathology of pulmonary hemorrhage and what role infiltrating neutrophils had in this process. Analysis of lung tissue from pristane-treated mice showed genes associated with ER stress and NETosis were increased in a time-dependent manner and reflected the timing of CD11b+Ly6G+ neutrophil accumulation in the lung. Using precision cut lung slices from untreated mice we observed that neutrophils isolated from the peritoneal cavity of pristane-treated mice could directly induce the expression of genes associated with ER stress, namely Chop and Bip. Mice which had myeloid-specific deletion of PAD4 were generated and treated with pristane to assess the involvement of PAD4 and PAD4-dependent NET formation in pristane-induced lung inflammation. Specific deletion of PAD4 in myeloid cells resulted in decreased expression of ER stress genes in the pristane model, with accompanying reduction in IFN-driven genes and pathology. Lastly, coculture experiments of human neutrophils and human lung epithelial cell line (BEAS-2b) showed neutrophils from SLE patients induced significantly more ER stress and interferon-stimulated genes in epithelial cells compared to healthy control neutrophils. These results support a pathogenic role of neutrophils and NETs in lung injury during pristane-induced DAH through the induction of ER stress response and suggest that overactivation of neutrophils in SLE and NETosis may underlie development of DAH.
Keywords: ER stress; NEtosis; diffuse alveolar hemorrhage (DAH); lung inflammation; neutrophil (PMN); systemic lupus erythematosus (SLE).
Copyright © 2022 Tumurkhuu, Laguna, Moore, Contreras, Santos, Akaveka, Montano, Wang, Ishimori, Venuturupalli, Forbess, Stripp, Wallace and Jefferies.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

