Robust and Functional Immune Memory Up to 9 Months After SARS-CoV-2 Infection: A Southeast Asian Longitudinal Cohort
- PMID: 35185909
- PMCID: PMC8853741
- DOI: 10.3389/fimmu.2022.817905
Robust and Functional Immune Memory Up to 9 Months After SARS-CoV-2 Infection: A Southeast Asian Longitudinal Cohort
Abstract
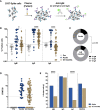
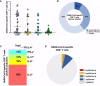
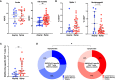
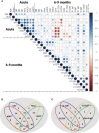
The duration of humoral and cellular immune memory following SARS-CoV-2 infection in populations in least developed countries remains understudied but is key to overcome the current SARS-CoV-2 pandemic. Sixty-four Cambodian individuals with laboratory-confirmed infection with asymptomatic or mild/moderate clinical presentation were evaluated for Spike (S)-binding and neutralizing antibodies and antibody effector functions during acute phase of infection and at 6-9 months follow-up. Antigen-specific B cells, CD4+ and CD8+ T cells were characterized, and T cells were interrogated for functionality at late convalescence. Anti-S antibody titers decreased over time, but effector functions mediated by S-specific antibodies remained stable. S- and nucleocapsid (N)-specific B cells could be detected in late convalescence in the activated memory B cell compartment and are mostly IgG+. CD4+ and CD8+ T cell immune memory was maintained to S and membrane (M) protein. Asymptomatic infection resulted in decreased antibody-dependent cellular cytotoxicity (ADCC) and frequency of SARS-CoV-2-specific CD4+ T cells at late convalescence. Whereas anti-S antibodies correlated with S-specific B cells, there was no correlation between T cell response and humoral immune memory. Hence, all aspects of a protective immune response are maintained up to nine months after SARS-CoV-2 infection and in the absence of re-infection.
Keywords: B cell immunity; SARS-CoV-2; T cell immunity; antibody effector function; long term immune response.
Copyright © 2022 Vo, Maestri, Auerswald, Sorn, Lay, Seng, Sann, Ya, Pean, Dussart, Schwartz, Ly, Bruel, Ly, Duong, Karlsson and Cantaert.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Characterization of SARS-CoV-2-Specific Humoral and Cellular Immune Responses Induced by Inactivated COVID-19 Vaccines in a Real-World Setting.Front Immunol. 2021 Dec 22;12:802858. doi: 10.3389/fimmu.2021.802858. eCollection 2021. Front Immunol. 2021. PMID: 35003131 Free PMC article.
-
Deciphering the Role of Humoral and Cellular Immune Responses in Different COVID-19 Vaccines-A Comparison of Vaccine Candidate Genes in Roborovski Dwarf Hamsters.Viruses. 2021 Nov 16;13(11):2290. doi: 10.3390/v13112290. Viruses. 2021. PMID: 34835096 Free PMC article.
-
Distinct immune memory induced by SARS-CoV-2 in convalescent liver transplant recipients.Front Immunol. 2025 Apr 2;16:1420150. doi: 10.3389/fimmu.2025.1420150. eCollection 2025. Front Immunol. 2025. PMID: 40242765 Free PMC article.
-
Phenotypes and Functions of SARS-CoV-2-Reactive T Cells.Mol Cells. 2021 Jun 30;44(6):401-407. doi: 10.14348/molcells.2021.0079. Mol Cells. 2021. PMID: 34120892 Free PMC article. Review.
-
Will SARS-CoV-2 Infection Elicit Long-Lasting Protective or Sterilising Immunity? Implications for Vaccine Strategies (2020).Front Immunol. 2020 Dec 9;11:571481. doi: 10.3389/fimmu.2020.571481. eCollection 2020. Front Immunol. 2020. PMID: 33362759 Free PMC article. Review.
Cited by
-
Persistence of T-Cell Immunity Responses against SARS-CoV-2 for over 12 Months Post COVID-19 Infection in Unvaccinated Individuals with No Detectable IgG Antibodies.Vaccines (Basel). 2023 Nov 27;11(12):1764. doi: 10.3390/vaccines11121764. Vaccines (Basel). 2023. PMID: 38140169 Free PMC article.
-
Cellular Immunity of SARS-CoV-2 in the Borriana COVID-19 Cohort: A Nested Case-Control Study.Epidemiologia (Basel). 2024 Apr 10;5(2):167-186. doi: 10.3390/epidemiologia5020012. Epidemiologia (Basel). 2024. PMID: 38651389 Free PMC article.
-
De novo design of anti-variant COVID-19 vaccine.Biol Methods Protoc. 2023 Sep 26;8(1):bpad021. doi: 10.1093/biomethods/bpad021. eCollection 2023. Biol Methods Protoc. 2023. PMID: 37854896 Free PMC article.
-
Individual Immune Response to SARS-CoV-2 Infection-The Role of Seasonal Coronaviruses and Human Leukocyte Antigen.Biology (Basel). 2023 Sep 28;12(10):1293. doi: 10.3390/biology12101293. Biology (Basel). 2023. PMID: 37887003 Free PMC article.
-
Preserved T cell but attenuated antibody response in MS patients on fingolimod and ocrelizumab following 2nd and 3rd SARS-CoV-2 mRNA vaccine.Mult Scler J Exp Transl Clin. 2023 Apr 5;9(2):20552173231165196. doi: 10.1177/20552173231165196. eCollection 2023 Apr-Jun. Mult Scler J Exp Transl Clin. 2023. PMID: 37057191 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

