Jianpi Qingchang Decoction Ameliorates Chronic Colitis in Piroxicam-Induced IL-10 Knockout Mice by Inhibiting Endoplasmic Reticulum Stress
- PMID: 35186102
- PMCID: PMC8849791
- DOI: 10.1155/2022/7378807
Jianpi Qingchang Decoction Ameliorates Chronic Colitis in Piroxicam-Induced IL-10 Knockout Mice by Inhibiting Endoplasmic Reticulum Stress
Abstract
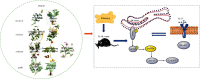
Background: Excessive endoplasmic reticulum (ER) stress in intestinal epithelial cells (IEC) may lead to impaired intestinal mucosal barrier function and then participate in the pathogenesis of ulcerative colitis (UC). Jianpi Qingchang decoction (JPQCD) has been shown to have protective effects on UC. However, further studies are needed to determine whether JPQCD regulates PERK/eIF2α/ATF4/CHOP pathways to play a role in treating UC.
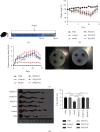
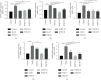
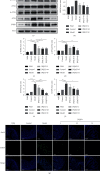
Methods: IL-10 -/- mice were randomly assigned into five groups: control, model, low-dose JPQCD (JPQCD L), middle-dose JPQCD (JPQCD M), and high-dose JPQCD (JPQCD H). All groups except for the control group were given model feed containing 200 ppm piroxicam for 10 d to induce colitis. As a comparison, we used wild-type mice that were the progeny of IL-10 +/- matings, bred in the same facility. The control group and wild-type mice were fed with common feed. At the same time, mice in each group were given corresponding drugs by gavage for 14 d. The disease activity index of mice in each group was evaluated daily. Colon tissues of mice were collected, colon length was measured, and pathological changes and ultrastructure of colon epithelial cells were observed. The effects of JPQCD on the PERK/eIF2α/ATF4/CHOP pathways were evaluated by western blotting and reverse transcription-polymerase chain reaction (RT-PCR). The expression of CHOP in colon tissue was detected by tissue immunofluorescence assay. The expression of NF-κB, p-NF-κB p65 protein was analyzed by western blotting; the level of IL-17 in colon tissue was detected by enzyme-linked immunosorbent assay (ELISA) and verified by examining NF-κB and IL-17 mRNA levels by RT-PCR.
Results: Compared with the control group, the model group showed significant colitis symptoms and severe colonic tissue damage. The results showed that JPQCD significantly reduced body weight loss, ameliorated disease activity index, and restored colon length in IL-10 -/- mice with piroxicam-induced colitis. Western blotting and RT-PCR showed that the PERK/eIF2α/ATF4/CHOP pathway was activated in colon tissue of model mice, suggesting that the pathway is involved in the pathogenesis of ulcerative colitis (UC) and could become a potential therapeutic target. The JPQCD treatment inhibited the activation of the PERK/eIF2α/ATF4/CHOP pathway, alleviated the ER stress, and played a role in preventing and treating UC. In addition, JPQCD can also downregulate the protein of NF-κB, p-NF-κB p65, downregulate the mRNA expression of NF-κB, and reduce the content of IL-17 and its mRNA expression in colon tissues.
Conclusion: JPQCD may play a protective role in UC by regulating the PERK/eIF2α/ATF4/CHOP signaling pathway and relieving endoplasmic reticulum stress.
Copyright © 2022 Qian Chen et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest related to this work.
Figures




Similar articles
-
Jianpi Qingchang decoction alleviates ulcerative colitis by inhibiting nuclear factor-κB activation.World J Gastroenterol. 2017 Feb 21;23(7):1180-1188. doi: 10.3748/wjg.v23.i7.1180. World J Gastroenterol. 2017. PMID: 28275298 Free PMC article.
-
Mesalazine alleviated the symptoms of spontaneous colitis in interleukin-10 knockout mice by regulating the STAT3/NF-κB signaling pathway.World J Gastroenterol. 2025 Feb 21;31(7):96459. doi: 10.3748/wjg.v31.i7.96459. World J Gastroenterol. 2025. PMID: 39991681 Free PMC article.
-
Cucurbitacin IIa Ameliorates DSS-Induced Ulcerative Colitis via Enhancing Intestinal Barrier Function and Inhibiting PERK/ATF4/CHOP Signaling Pathway.Turk J Gastroenterol. 2024 Dec 23;36(4):229-240. doi: 10.5152/tjg.2024.24449. Turk J Gastroenterol. 2024. PMID: 39763247 Free PMC article.
-
Excessive Apoptosis in Ulcerative Colitis: Crosstalk Between Apoptosis, ROS, ER Stress, and Intestinal Homeostasis.Inflamm Bowel Dis. 2022 Mar 30;28(4):639-648. doi: 10.1093/ibd/izab277. Inflamm Bowel Dis. 2022. PMID: 34871402 Review.
-
Mechanism of Qingchang Suppository on repairing the intestinal mucosal barrier in ulcerative colitis.Front Pharmacol. 2023 Aug 22;14:1221849. doi: 10.3389/fphar.2023.1221849. eCollection 2023. Front Pharmacol. 2023. PMID: 37675045 Free PMC article. Review.
Cited by
-
Clinical study of Jianpi Qingchang decoction in the treatment of ulcerative colitis patients with spleen deficiency and dampness-heat syndrome accompanied by fatigue: Study protocol for a randomized controlled trial.Contemp Clin Trials Commun. 2024 Dec 5;42:101409. doi: 10.1016/j.conctc.2024.101409. eCollection 2024 Dec. Contemp Clin Trials Commun. 2024. PMID: 39735170 Free PMC article.
-
Qing Hua Chang Yin ameliorates chronic colitis in mice by inhibiting PERK-ATF4-CHOP pathway of ER stress and the NF-κB signalling pathway.Pharm Biol. 2024 Dec;62(1):607-620. doi: 10.1080/13880209.2024.2378012. Epub 2024 Jul 22. Pharm Biol. 2024. PMID: 39034914 Free PMC article.
-
Biogenic Selenium Nanoparticles Alleviate Intestinal Epithelial Barrier Damage through Regulating Endoplasmic Reticulum Stress-Mediated Mitophagy.Oxid Med Cell Longev. 2022 Aug 5;2022:3982613. doi: 10.1155/2022/3982613. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36035212 Free PMC article.
References
-
- Yeshi K., Ruscher R., Hunter L., Daly N. L., Loukas A., Wangchuk P. Revisiting inflammatory bowel disease: pathology, treatments, challenges and emerging therapeutics including drug leads from natural products. Journal of Clinical Medicine . 2020;9(5):p. 1273. doi: 10.3390/jcm9051273. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

