miR-488-3p Protects Cardiomyocytes against Doxorubicin-Induced Cardiotoxicity by Inhibiting CyclinG1
- PMID: 35186188
- PMCID: PMC8853758
- DOI: 10.1155/2022/5184135
miR-488-3p Protects Cardiomyocytes against Doxorubicin-Induced Cardiotoxicity by Inhibiting CyclinG1
Abstract
Objective: To investigate the protective effects and regulatory mechanism of miR-488-3p on doxorubicin-induced cardiotoxicity.
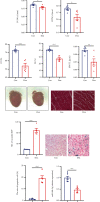
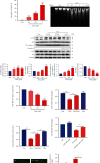
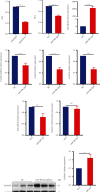
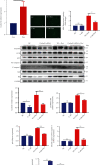
Methods: The C57BL/6 mice and primary cardiomyocytes were used to construct doxorubicin-induced cardiomyocyte injury models in vivo and in vitro. The levels of miR-488-3p and its downstream target genes were analyzed by quantitative real-time PCR. Mouse cardiac function, cell survival, cellular injury-related proteins, and the apoptosis level of cardiomyocytes were analyzed by echocardiography, MTT analysis, Western blotting, and DNA laddering separately.
Results: Cardiomyocyte injury caused by a variety of stimuli can lead to the reduction of miR-488-3p level, especially when stimulated with doxorubicin. Doxorubicin led to significant decrease in cardiac function, cell autophagic flux blockage, and apoptosis in vivo and in vitro. The expression of miR-488-3p's target gene, CyclinG1, increased remarkably in the doxorubicin-treated neonatal mouse cardiomyocytes. Overexpression of miR-488-3p inhibited CyclinG1 expression, increased cardiomyocyte viability, and attenuated doxorubicin-induced cardiomyocyte autophagic flux blockage and apoptosis.
Conclusions: miR-488-3p is one of the important protective miRNAs in doxorubicin-induced cardiotoxicity by inhibiting the expression of CyclinG1, which provides insight into the possible clinical application of miR-488-3p/CyclinG1 as therapeutic targets in doxorubicin-induced cardiovascular diseases.
Copyright © 2022 Mingjing Yan et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures

Similar articles
-
Upregulation of let-7f-2-3p by long noncoding RNA NEAT1 inhibits XPO1-mediated HAX-1 nuclear export in both in vitro and in vivo rodent models of doxorubicin-induced cardiotoxicity.Arch Toxicol. 2019 Nov;93(11):3261-3276. doi: 10.1007/s00204-019-02586-4. Epub 2019 Sep 30. Arch Toxicol. 2019. PMID: 31570982
-
Dexrazoxane Protects Cardiomyocyte from Doxorubicin-Induced Apoptosis by Modulating miR-17-5p.Biomed Res Int. 2020 Mar 1;2020:5107193. doi: 10.1155/2020/5107193. eCollection 2020. Biomed Res Int. 2020. PMID: 32190669 Free PMC article.
-
MiR-24-3p Attenuates Doxorubicin-induced Cardiotoxicity via the Nrf2 Pathway in Mice.Curr Med Sci. 2022 Feb;42(1):48-55. doi: 10.1007/s11596-022-2536-1. Epub 2022 Jan 28. Curr Med Sci. 2022. PMID: 35089495
-
Autophagy and mitophagy in the context of doxorubicin-induced cardiotoxicity.Oncotarget. 2017 Jul 11;8(28):46663-46680. doi: 10.18632/oncotarget.16944. Oncotarget. 2017. PMID: 28445146 Free PMC article. Review.
-
Doxorubicin-Induced Cardiomyopathy in Children.Compr Physiol. 2019 Jun 12;9(3):905-931. doi: 10.1002/cphy.c180017. Compr Physiol. 2019. PMID: 31187890 Free PMC article. Review.
Cited by
-
A recent decade update on combating doxorubicin-induced toxicities.Arch Toxicol. 2025 Sep;99(9):3565-3578. doi: 10.1007/s00204-025-04112-1. Epub 2025 Jul 2. Arch Toxicol. 2025. PMID: 40600983 Review.
-
Doxorubicin-Induced Cardiotoxicity: A Comprehensive Update.J Cardiovasc Dev Dis. 2025 May 30;12(6):207. doi: 10.3390/jcdd12060207. J Cardiovasc Dev Dis. 2025. PMID: 40558642 Free PMC article. Review.
-
Clinical Significance of Non-Coding RNA Regulation of Programmed Cell Death in Hepatocellular Carcinoma.Cancers (Basel). 2023 Aug 21;15(16):4187. doi: 10.3390/cancers15164187. Cancers (Basel). 2023. PMID: 37627215 Free PMC article. Review.
-
Effect of an NGR Peptide on the Efficacy of the Doxorubicin Phospholipid Delivery System.Nanomaterials (Basel). 2023 Aug 1;13(15):2229. doi: 10.3390/nano13152229. Nanomaterials (Basel). 2023. PMID: 37570547 Free PMC article.
-
Screening and Evaluation of tRF-Glu-CTC-013 as a Biomarker and Key Regulator in the Development of Cardiac Hypertrophy.Int J Med Sci. 2025 Jun 9;22(11):2839-2851. doi: 10.7150/ijms.106114. eCollection 2025. Int J Med Sci. 2025. PMID: 40520901 Free PMC article.
References
-
- Kocarnik J. M., Compton K., Dean F. E., et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. JAMA Oncology . 2021 doi: 10.1001/jamaoncol.2021.6987. - DOI - PMC - PubMed
-
- Kirkham A. A., Eves N. D., Shave R. E., et al. The effect of an aerobic exercise bout 24 h prior to each doxorubicin treatment for breast cancer on markers of cardiotoxicity and treatment symptoms: a RCT. Breast Cancer Research and Treatment . 2018;167(3):719–729. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

