Pharmacological management of severe Cushing's syndrome: the role of etomidate
- PMID: 35186251
- PMCID: PMC8848075
- DOI: 10.1177/20420188211058583
Pharmacological management of severe Cushing's syndrome: the role of etomidate
Abstract
Cushing's syndrome (CS) is an endocrine disease characterized by excessive adrenocortical steroid production. One of the mainstay pharmacological treatments for CS are steroidogenesis enzyme inhibitors, including the antifungal agent ketoconazole along with metyrapone, mitotane, and aminoglutethimide. Recently, osilodrostat was added to this drug class and approved by the US Food and Drug Administration (FDA) for the treatment of Cushing's Disease. Steroidogenesis enzyme inhibitors inhibit various enzymes along the cortisol biosynthetic pathway and may be used preoperatively to lower cortisol levels and reduce surgical risk associated with tumor resection or postoperatively when surgery and/or radiation therapies are not curative. Because their selectivities for steroidogenic enzymes vary, they may even be administered in combination to achieve relatively rapid control of severe hypercortisolemia. Unfortunately, all currently available inhibitors are accompanied by serious adverse side effects that limit dosing and often result in treatment failures. Although more commonly known as a general anesthetic induction agent, etomidate is another member of the steroidogenesis enzyme inhibitor drug class. It suppresses cortisol production primarily by inhibiting 11β-hydroxylase and is the only inhibitor that may be given parenterally. However, the sedative-hypnotic actions of etomidate limit its use as an acute management option for CS. Thus, some have recommended that it be used only in intensive care settings. In this review, we discuss the initial development of etomidate as an anesthetic agent, its subsequent development as a treatment for CS, and the recent advances in dosing and drug development that dissociate sedative-hypnotic and adrenostatic drug actions to facilitate CS treatment in non-critical care settings.
Keywords: Cushing’s syndrome; corticosterone; cortisol; etomidate.
© The Author(s), 2022.
Conflict of interest statement
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Raines is the inventor of patented technologies involving the design of etomidate analogs that potently inhibit cortisol production but lack sedative-hypnotic activity.
Figures
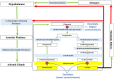
Similar articles
-
Therapy of Cushing's syndrome with steroid biosynthesis inhibitors.J Steroid Biochem Mol Biol. 1994 Jun;49(4-6):261-7. doi: 10.1016/0960-0760(94)90267-4. J Steroid Biochem Mol Biol. 1994. PMID: 8043488 Review.
-
Cushing's syndrome: a combined treatment with etomidate and osilodrostat in severe life-threatening hypercortisolemia.Hormones (Athens). 2022 Dec;21(4):735-742. doi: 10.1007/s42000-022-00397-4. Epub 2022 Sep 21. Hormones (Athens). 2022. PMID: 36129663 Free PMC article. Review.
-
Dimethoxy-etomidate: A Nonhypnotic Etomidate Analog that Potently Inhibits Steroidogenesis.J Pharmacol Exp Ther. 2018 Feb;364(2):229-237. doi: 10.1124/jpet.117.245332. Epub 2017 Dec 4. J Pharmacol Exp Ther. 2018. PMID: 29203576 Free PMC article.
-
Updates in adrenal steroidogenesis inhibitors for Cushing's syndrome - A practical guide.Best Pract Res Clin Endocrinol Metab. 2021 Jan;35(1):101490. doi: 10.1016/j.beem.2021.101490. Epub 2021 Feb 6. Best Pract Res Clin Endocrinol Metab. 2021. PMID: 33707082 Review.
-
Drugs in the medical treatment of Cushing's syndrome.Expert Opin Emerg Drugs. 2009 Dec;14(4):661-71. doi: 10.1517/14728210903413522. Expert Opin Emerg Drugs. 2009. PMID: 19939210 Review.
Cited by
-
Cushing syndrome.Nat Rev Dis Primers. 2025 Jan 23;11(1):4. doi: 10.1038/s41572-024-00588-w. Nat Rev Dis Primers. 2025. PMID: 39848955 Review.
-
Etomidate-induced hypokalemia in electronic cigarette users: two case reports and literature review.Front Endocrinol (Lausanne). 2024 May 30;15:1321610. doi: 10.3389/fendo.2024.1321610. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38872965 Free PMC article. Review.
-
Individualized medical treatment options in Cushing disease.Front Endocrinol (Lausanne). 2022 Dec 2;13:1060884. doi: 10.3389/fendo.2022.1060884. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36531477 Free PMC article. Review.
-
Approaches based on natural products and miRNAs in pituitary adenomas: unveiling therapeutic intervention.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jan;398(1):69-88. doi: 10.1007/s00210-024-03347-6. Epub 2024 Aug 5. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39102032 Review.
-
Effects of spinal-epidural anesthesia combined with intravenous etomidate on adrenocortical and immune stress in elderly patients undergoing anorectal surgery: A retrospective analysis.Biomol Biomed. 2025 Jan 30;25(3):701-707. doi: 10.17305/bb.2024.10759. Biomol Biomed. 2025. PMID: 39097834 Free PMC article. Review.
References
-
- Fernandez-Rodriguez E, Stewart PM, Cooper MS. The pituitary-adrenal axis and body composition. Pituitary 2009; 12: 105–115. - PubMed
-
- Pereira AM, Tiemensma J, Romijn JA. Neuropsychiatric disorders in Cushing’s syndrome. Neuroendocrinology 2010; 92(Suppl. 1): 65–70. - PubMed
-
- Hinojosa-Amaya JM, Cuevas-Ramos D, Fleseriu M. Medical management of Cushing’s syndrome: current and emerging treatments. Drugs 2019; 79: 935–956. - PubMed
-
- Wengander S, Trimpou P, Papakokkinou E, et al.. The incidence of endogenous Cushing’s syndrome in the modern era. Clin Endocrinol 2019; 91: 263–270. - PubMed
-
- Feelders RA, Hofland LJ, de Herder WW. Medical treatment of Cushing’s syndrome: adrenal-blocking drugs and ketaconazole. Neuroendocrinology 2010; 92(Suppl. 1): 111–115. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

