COVID-19 and Liver Dysfunction
- PMID: 35186564
- PMCID: PMC8849487
- DOI: 10.7759/cureus.21302
COVID-19 and Liver Dysfunction
Abstract
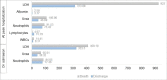
Introduction The pandemic of coronavirus disease 2019 (COVID-19) has caused over four million deaths, depleting resources and healthcare workers; therefore, in an attempt to stratify patients, the relationship between liver enzymes and clinical outcome was studied. This study aimed to assess the pattern and impact of liver enzymes on the clinical outcome of hospitalized patients with COVID-19 in Lebanon and look for possible confounding factors. Methodology This was a single-centered retrospective cohort study conducted between December 2020 and March 2021 on 230 patients diagnosed with COVID-19. Liver function tests (LFTs) and other laboratory values on admission and peak hospitalization were analyzed using SPSS. Results The prevalence of abnormal liver tests among the sample population with severe COVID-19 infection were as follows: aspartate aminotransferase (AST), 77%; alanine aminotransferase (ALT), 49%; alkaline phosphatase (ALP), 12%; and gamma-glutamyl transferase (GGT), 37%. A severe COVID-19 infection was more likely present in patients with abnormal levels of AST (p = 0.015), ALP (p = 0.03), and GGT (p = 0.022). ANOVA test revealed no significant relationship between AST levels at peak hospitalization and the treatments received by the patient. Conclusion Abnormal liver function tests of patients at admission may be an indicator of more severe disease. In the context of scarce resources created by the pandemic, it becomes essential to establish a reliable predictor for a severe outcome of COVID-19 infection and manage accordingly.
Keywords: cholestatic; coronavirus; hepatocellular; hepatotoxic; liver function tests.
Copyright © 2022, Ibrahim et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Monitoring of COVID-19 infection in Lebanon. [ Dec; 2021 ];https://www.moph.gov.lb/en/DynamicPages/index/127/43750/monitoring-of-co... 2021
-
- Extrapulmonary clinical manifestations in COVID-19 patients. Sarkesh A, Daei Sorkhabi A, Sheykhsaran E, Alinezhad F, Mohammadzadeh N, Hemmat N, Bannazadeh Baghi H. https://doi.org/10.4269/ajtmh.20-0986. Am J Trop Med Hyg. 2020;103:1783–1796. - PMC - PubMed
-
- Extrapulmonary complications of COVID-19: a multisystem disease? Zheng KI, Feng G, Liu WY, Targher G, Byrne CD, Zheng MH. https://doi.org/10.1002/jmv.26294. J Med Virol. 2021;93:323–335. - PMC - PubMed
-
- COVID-19-associated gastrointestinal and liver injury: clinical features and potential mechanisms. Zhong P, Xu J, Yang D, et al. https://doi.org/10.1038/s41392-020-00373-7. Signal Transduct Target Ther. 2020;5:256. - PMC - PubMed
-
- Abnormal liver function tests predict transfer to intensive care unit and death in COVID-19. Piano S, Dalbeni A, Vettore E, et al. https://doi.org/10.1111/liv.14565. Liver Int. 2020;40:2394–2406. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous