Prevention of Oxaliplatin-Induced Peripheral Neuropathy: A Systematic Review and Meta-Analysis
- PMID: 35186722
- PMCID: PMC8853097
- DOI: 10.3389/fonc.2022.731223
Prevention of Oxaliplatin-Induced Peripheral Neuropathy: A Systematic Review and Meta-Analysis
Abstract
Background: Oxaliplatin-induced peripheral neuropathy (OIPN) has significant clinical impact on the quality of life for cancer patients and is a dose limiting toxicity. Trials studying preventive measures have been inconclusive. A systematic review and meta-analysis were conducted to evaluate the existing pharmacological and non-pharmacological interventions to prevent chronic OIPN.
Methods: Literature databases PubMed-MEDLINE, Embase and Scopus, were searched from 1 Jan 2005 to 08 Aug 2020 and major conferences' abstracts were reviewed for randomized controlled trials that examined the efficacy of any preventive measure for OIPN. The primary outcome measure was the incidence of chronic OIPN with a preventive intervention as compared to placebo or no intervention. The pooled risk ratio and its 95% confidence interval were calculated using a random effects model. A network meta-analysis was conducted to derive indirect evidence of any preventive effect of an intervention against placebo when original trials compared one intervention against another.
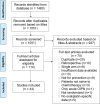
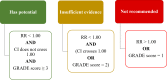
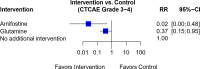
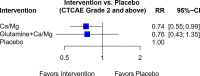
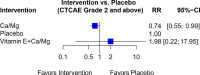
Results: Forty-four trials were analyzed describing 29 chemoprotective interventions, including combinations, and 1 non-pharmacological intervention. Ratings were assessed via a combination of outcomes with quality assessment using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework. Of the 30 interventions examined, there were six interventions supporting potential efficacy, 11 interventions with insufficient evidence and 13 interventions not recommended.
Conclusion: Currently, there is insufficient certainty to support any intervention as effective in preventing OIPN. Of note is that most of these studies have focused on pharmacological interventions; non-pharmacological interventions are underexplored. Further research on ways to limit OIPN is needed.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=225095, Prospero Registration Number: CRD42021225095.
Keywords: meta-analysis; network analysis; neurotoxicity; non-pharmacological; oxaliplatin; peripheral neuropathy; pharmacological.
Copyright © 2022 Peng, Ying, Chan, Sundar, Soon and Bandla.
Conflict of interest statement
RS is a member on the advisory board of Bristol Myers Squibb, Merck, Eisai, Bayer, Taiho, Novartis, MSD. He has received honoraria for talks from MSD, Eli Lilly, BMS, Roche, Taiho, Astra Zeneca. He has received travel funding from Paxman Coolers Ltd., Roche, Astra Zeneca, Taiho, Eisai. He has received research funding from Paxman Coolers, MSD. AB has received travel funding from Paxman Coolers Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Loprinzi CL, Qin R, Dakhil SR, Fehrenbacher L, Flynn KA, Atherton P, et al. . Phase III Randomized, Placebo-Controlled, Double-Blind Study of Intravenous Calcium and Magnesium to Prevent Oxaliplatin-Induced Sensory Neurotoxicity (N08CB/Alliance). J Clin Oncol (2014) 32(10):997–1005. doi: 10.1200/JCO.2013.52.0536 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

