A Jack of All Trades: The Role of Pneumococcal Surface Protein A in the Pathogenesis of Streptococcus pneumoniae
- PMID: 35186799
- PMCID: PMC8847780
- DOI: 10.3389/fcimb.2022.826264
A Jack of All Trades: The Role of Pneumococcal Surface Protein A in the Pathogenesis of Streptococcus pneumoniae
Abstract
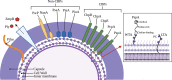
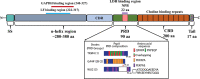
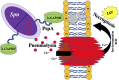
Streptococcus pneumoniae (Spn), or the pneumococcus, is a Gram-positive bacterium that colonizes the upper airway. Spn is an opportunistic pathogen capable of life-threatening disease should it become established in the lungs, gain access to the bloodstream, or disseminate to vital organs including the central nervous system. Spn is encapsulated, allowing it to avoid phagocytosis, and current preventative measures against infection include polyvalent vaccines composed of capsular polysaccharide corresponding to its most prevalent serotypes. The pneumococcus also has a plethora of surface components that allow the bacteria to adhere to host cells, facilitate the evasion of the immune system, and obtain vital nutrients; one family of these are the choline-binding proteins (CBPs). Pneumococcal surface protein A (PspA) is one of the most abundant CBPs and confers protection against the host by inhibiting recognition by C-reactive protein and neutralizing the antimicrobial peptide lactoferricin. Recently our group has identified two new roles for PspA: binding to dying host cells via host-cell bound glyceraldehyde 3-phosphate dehydrogenase and co-opting of host lactate dehydrogenase to enhance lactate availability. These properties have been shown to influence Spn localization and enhance virulence in the lower airway, respectively. Herein, we review the impact of CBPs, and in particular PspA, on pneumococcal pathogenesis. We discuss the potential and limitations of using PspA as a conserved vaccine antigen in a conjugate vaccine formulation. PspA is a vital component of the pneumococcal virulence arsenal - therefore, understanding the molecular aspects of this protein is essential in understanding pneumococcal pathogenesis and utilizing PspA as a target for treating or preventing pneumococcal pneumonia.
Keywords: Streptococcus pneumoniae; choline-binding proteins; pathogenesis; pneumococcal surface protein A (PspA); vaccine.
Copyright © 2022 Lane, Tata, Briles and Orihuela.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Askim Å., Mehl A., Paulsen J., Dewan A. T., Vestrheim D. F., Åsvold B. O., et al. (2016). Epidemiology and Outcome of Sepsis in Adult Patients With Streptococcus pneumoniae Infection in a Norwegian County 1993–2011: An Observational Study. BMC Infect. Dis. 16, 223. doi: 10.1186/s12879-016-1553-8 - DOI - PMC - PubMed
-
- Asmat T. M., Agarwal V., Saleh M., Hammerschmidt S. (2014). Endocytosis of Streptococcus pneumoniae via the Polymeric Immunoglobulin Receptor of Epithelial Cells Relies on Clathrin and Caveolin Dependent Mechanisms. Int. J. Med. Microbiol. 304, 1233–1246. doi: 10.1016/j.ijmm.2014.10.001 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

