Profiling Selective Packaging of Host RNA and Viral RNA Modification in SARS-CoV-2 Viral Preparations
- PMID: 35186917
- PMCID: PMC8851031
- DOI: 10.3389/fcell.2022.768356
Profiling Selective Packaging of Host RNA and Viral RNA Modification in SARS-CoV-2 Viral Preparations
Abstract
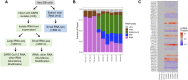
Viruses package host RNAs in their virions which are associated with a range of functions in the viral life cycle. Previous transcriptomic profiling of host RNA packaging mostly focused on retroviruses. Which host RNAs are packaged in other viruses at the transcriptome level has not been thoroughly examined. Here we perform proof-of-concept studies using both small RNA and large RNA sequencing of six different SARS-CoV-2 viral isolates grown on VeroE6 cells to profile host RNAs present in cell free viral preparations and to explore SARS-CoV-2 genomic RNA modifications. We find selective enrichment of specific host transfer RNAs (tRNAs), tRNA fragments and signal recognition particle (SRP) RNA in SARS-CoV-2 viral preparations. Different viral preparations contain the same set of host RNAs, suggesting a common mechanism of packaging. We estimate that a single SARS-CoV-2 particle likely contains up to one SRP RNA and four tRNA molecules. We identify tRNA modification differences between the tRNAs present in viral preparations and those in the uninfected VeroE6 host cells. Furthermore, we find uncharacterized candidate modifications in the SARS-CoV-2 genomic RNA. Our results reveal an under-studied aspect of viral-host interactions that may be explored for viral therapeutics.
Keywords: SARS-CoV-2; SRP RNA; modification; packaging; tRNA.
Copyright © 2022 Peña, Zhang, Watkins, Halucha, Alshammary, Hernandez, Liu, Albrecht, Garcia-Sastre, Simon, Katanski and Pan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

