Low Bone Mineral Density in Hemophiliacs
- PMID: 35186990
- PMCID: PMC8849249
- DOI: 10.3389/fmed.2022.794456
Low Bone Mineral Density in Hemophiliacs
Abstract
Objective: To review the current knowledge on bone health in patients with hemophilia A and the underlying pathogenetic mechanisms.
Data sources: Original research articles, meta-analyses, and scientific reviews.
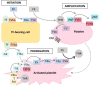
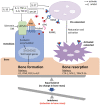
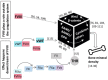
Data synthesis: Already in childhood, patients with hemophilia A are prone to low bone mineral density, leading to osteopenia and/or osteoporosis. Initially associated with the life style of hemophilia, today we are faced with accumulating evidence that coagulation factor VIII is involved directly or indirectly in bone physiology.
Conclusion: Understanding the role of factor VIII and the mechanisms of decreased bone mineral density in hemophilia A is critically important, especially as non-factor replacement therapies are available, and treatment decisions potentially impact bone health.
Keywords: bone mineral density; coagulation; factor VIII; hemophilia A; osteopenia; osteoporosis.
Copyright © 2022 Gebetsberger, Schirmer, Wurzer and Streif.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Chaudhry R, Babiker HM. Physiology, Coagulation Pathways. In StatPearls. Treasure Island, FL: StatPearls Publishing; (2018). - PubMed
Publication types
LinkOut - more resources
Full Text Sources

