HTLV-1 and Co-infections
- PMID: 35187000
- PMCID: PMC8850362
- DOI: 10.3389/fmed.2022.812016
HTLV-1 and Co-infections
Abstract
Human T lymphotropic virus type 1 (HTLV-1) is a retrovirus that causes lifelong T-cell infection in humans, impacting the host immune response. This virus causes a range of clinical manifestations, from inflammatory conditions, including neuronal damage (HTLV-1 associated myelopathy, HAM) to life-threatening leukemia (adult T-cell leukemia, ATL). Human T lymphotropic virus type 1 is also associated with increased risk of all-cause mortality, but the mechanisms remain unclear. As a blood-borne and sexually transmitted infection (STI), HTLV-1 shares transmission routes to many other pathogens and although it has worldwide distribution, it affects mainly those in low- and middle-income tropical areas, where the prevalence of other infectious agents is high. These factors contribute to a high incidence of co-infections in people living with HTLV-1 (PLHTLV). This comprehensive review addresses the impact of HTLV-1 on several co-infections and vice-versa. There is evidence of higher rates of HTLV-1 infection in association with other blood borne (HCV, HBV) and sexually transmitted (Syphilis, Chlamydia, HPV, HSV) infections but whether this represents increased susceptibility or opportunity is unclear. Higher frequency of Mycobacterium tuberculosis (MTb) and Mycobacterium leprae (M. leprae) is observed in PLHTLV. Reports of opportunistic infections and high frequency of crusted scabies in patients with HTLV-1 points to immune impairment in those individuals. Human T lymphotropic virus type 1 may influence the persistence of pathogens, exemplified by the higher rates of Schistosoma mansoni and Strongyloides stercoralis (St. stercoralis) treatment failure observed in PLHTLV. This retrovirus is also associated with increased tuberculosis (TB) severity with some evidence pointing to a deleterious impact on leprosy outcome as well. These findings are supported by immune alterations observed in those co-infected individuals. Although the role of HTLV-1 in HCV outcome is debatable, most data indicate that HTLV may negatively impact the clinical course of hepatitis C. Co-infections may also influence the risk of developing HTLV-1 associated disease, but data are still limited. The impact of HTLV-1 on the response to more common infections, might contribute to the increased mortality rate of HTLV-1. Large scale prospective controlled studies on the prevalence and impact of HTLV-1 in co-infections and vice-versa are needed. Human T lymphotropic virus type 1 impact in public health is broad. Measures to increase awareness and to prevent new infections are needed.
Keywords: HBV; HCV; HTLV-1; Mycobacterium leprae; Mycobacterium tuberculosis; Schistosoma mansoni; co-infection; sexually transmitted infections.
Copyright © 2022 Rosadas and Taylor.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





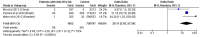

References
-
- Kawano N, Nagahiro Y, Yoshida S, Tahara Y, Himeji D, Kuriyama T, et al. . Clinical features and treatment outcomes of opportunistic infections among human T-lymphotrophic virus type 1 (HTLV-1) carriers and patients with adult T-cell leukemia-lymphoma (ATL) at a single institution from 2006 to 2016. J Clin Exp Hematop. (2019) 59:156–67. 10.3960/jslrt.18032 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

