Autoimmune Pemphigus: Latest Advances and Emerging Therapies
- PMID: 35187073
- PMCID: PMC8855930
- DOI: 10.3389/fmolb.2021.808536
Autoimmune Pemphigus: Latest Advances and Emerging Therapies
Abstract
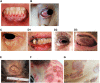
Pemphigus represents a group of rare and severe autoimmune intra-epidermal blistering diseases affecting the skin and mucous membranes. These painful and debilitating diseases are driven by the production of autoantibodies that are mainly directed against the desmosomal adhesion proteins, desmoglein 3 (Dsg3) and desmoglein 1 (Dsg1). The search to define underlying triggers for anti-Dsg-antibody production has revealed genetic, environmental, and possible vaccine-driven factors, but our knowledge of the processes underlying disease initiation and pathology remains incomplete. Recent studies point to an important role of T cells in supporting auto-antibody production; yet the involvement of the myeloid compartment remains unexplored. Clinical management of pemphigus is beginning to move away from broad-spectrum immunosuppression and towards B-cell-targeted therapies, which reduce many patients' symptoms but can have significant side effects. Here, we review the latest developments in our understanding of the predisposing factors/conditions of pemphigus, the underlying pathogenic mechanisms, and new and emerging therapies to treat these devastating diseases.
Keywords: advance in pemphigus; autoimmune bullous diseases; autoimmunity; pemphigus; pemphigus treatment.
Copyright © 2022 Lim, Bohelay, Hanakawa, Musette and Janela.
Conflict of interest statement
BJ receives research funding from Evonik. PM is a consultant for Servier. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

