Liquid-Liquid Phase Separation of TDP-43 and FUS in Physiology and Pathology of Neurodegenerative Diseases
- PMID: 35187086
- PMCID: PMC8847598
- DOI: 10.3389/fmolb.2022.826719
Liquid-Liquid Phase Separation of TDP-43 and FUS in Physiology and Pathology of Neurodegenerative Diseases
Abstract
Liquid-liquid phase separation of RNA-binding proteins mediates the formation of numerous membraneless organelles with essential cellular function. However, aberrant phase transition of these proteins leads to the formation of insoluble protein aggregates, which are pathological hallmarks of neurodegenerative diseases including ALS and FTD. TDP-43 and FUS are two such RNA-binding proteins that mislocalize and aggregate in patients of ALS and FTD. They have similar domain structures that provide multivalent interactions driving their phase separation in vitro and in the cellular environment. In this article, we review the factors that mediate and regulate phase separation of TDP-43 and FUS. We also review evidences that connect the phase separation property of TDP-43 and FUS to their functional roles in cells. Aberrant phase transition of TDP-43 and FUS leads to protein aggregation and disrupts their regular cell function. Therefore, restoration of functional protein phase of TDP-43 and FUS could be beneficial for neuronal cells. We discuss possible mechanisms for TDP-43 and FUS aberrant phase transition and aggregation while reviewing the methods that are currently being explored as potential therapeutic strategies to mitigate aberrant phase transition and aggregation of TDP-43 and FUS.
Keywords: ALS; FUS; TDP-43; liquid-liquid phase separation; stress granules.
Copyright © 2022 Carey and Guo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
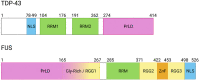

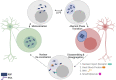
References
-
- Altman T., Ionescu A., Ibraheem A., Priesmann D., Gradus-Pery T., Farberov L., et al. (2021). Axonal TDP-43 Condensates Drive Neuromuscular junction Disruption through Inhibition of Local Synthesis of Nuclear Encoded Mitochondrial Proteins. Nat. Commun. 12, 6914. 10.1038/s41467-021-27221-8 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

