A Large Animal Model for Orthopedic Foot and Ankle Research
- PMID: 35187145
- PMCID: PMC8850350
- DOI: 10.3389/fvets.2022.816529
A Large Animal Model for Orthopedic Foot and Ankle Research
Abstract
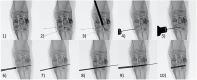
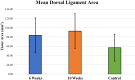
Trauma to the soft tissues of the ankle joint distal syndesmosis often leads to syndesmotic instability, resulting in undesired movement of the talus, abnormal pressure distributions, and ultimately arthritis if deterioration progresses without treatment. Historically, syndesmotic injuries have been repaired by placing a screw across the distal syndesmosis to provide rigid fixation to facilitate ligament repair. While rigid syndesmotic screw fixation immobilizes the ligamentous injury between the tibia and fibula to promote healing, the same screws inhibit normal physiologic movement and dorsiflexion. It has been shown that intact screw removal can be beneficial for long-term patient success; however, the exact timing remains an unanswered question that necessitates further investigation, perhaps using animal models. Because of the sparsity of relevant preclinical models, the purpose of this study was to develop a new, more translatable, large animal model that can be used for the investigation of clinical foot and ankle implants. Eight (8) skeletally mature sheep underwent stabilization of the left and right distal carpal bones following transection of the dorsal and interosseous ligaments while the remaining two animals served as un-instrumented controls. Four of the surgically stabilized animals were sacrificed 6 weeks after surgery while the remaining four animals were sacrificed 10 weeks after surgery. Ligamentous healing was evaluated using radiography, histology, histomorphometry, and histopathology. Overall, animals demonstrated a high tolerance to the surgical procedure with minimal complications. Animals sacrificed at 10 weeks post-surgery had a slight trend toward mildly decreased inflammation, decreased necrotic debris, and a slight increase in the healing of the transected ligaments. The overall degree of soft tissue fibrosis/fibrous expansion, including along the dorsal periosteal surfaces/joint capsule of the carpal bones was very similar between both timepoints and often exhibited signs of healing. The findings of this study indicate that the carpometacarpal joint may serve as a viable location for the investigation of human foot and ankle orthopedic devices. Future work may include the investigation of orthopedic foot and ankle medical devices, biologic treatments, and repair techniques in a large animal model capable of providing translational results for human treatment.
Keywords: carpus; fixation; foot and ankle; sheep; syndesmosis.
Copyright © 2022 Gadomski, Labus, Stewart, Bisazza, Nelson, Puttlitz, McGilvray, Regan and Easley.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
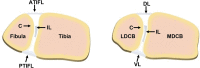


Similar articles
-
Anatomy of the distal tibiofibular syndesmosis in adults: a pictorial essay with a multimodality approach.J Anat. 2010 Dec;217(6):633-45. doi: 10.1111/j.1469-7580.2010.01302.x. J Anat. 2010. PMID: 21108526 Free PMC article. Review.
-
[Does removal of the syndesmotic screw improve clinical results of operative treatment of ankle fractures with concomitant syndesmosis injury?].Chir Narzadow Ruchu Ortop Pol. 2010 May-Jun;75(3):143-6. Chir Narzadow Ruchu Ortop Pol. 2010. PMID: 21038630 Polish.
-
The Fate of the Fixed Syndesmosis Over Time.Foot Ankle Int. 2015 Oct;36(10):1202-8. doi: 10.1177/1071100715588186. Epub 2015 Jun 3. Foot Ankle Int. 2015. PMID: 26041545
-
Anterior-inferior tibiofibular ligament anatomical repair and augmentation versus trans-syndesmosis screw fixation for the syndesmotic instability in external-rotation type ankle fracture with posterior malleolus involvement: A prospective and comparative study.Injury. 2016 Jul;47(7):1574-80. doi: 10.1016/j.injury.2016.04.014. Epub 2016 Apr 21. Injury. 2016. PMID: 27129908 Clinical Trial.
-
[Injuries of the inferior tibiofibular syndesmosis].Unfallchirurg. 2000 Jul;103(7):520-32. Unfallchirurg. 2000. PMID: 10969538 Review. German.
Cited by
-
Nasal anatomy and sniffing in respiration and olfaction of wild and domestic animals.Front Vet Sci. 2023 Jul 14;10:1172140. doi: 10.3389/fvets.2023.1172140. eCollection 2023. Front Vet Sci. 2023. PMID: 37520001 Free PMC article. Review.
-
Correction of syndesmotic malreduction following fixation flexibilization.Sci Rep. 2025 Jul 1;15(1):22434. doi: 10.1038/s41598-025-04117-x. Sci Rep. 2025. PMID: 40593952 Free PMC article.
References
-
- Bonnel FBM, Canovas F, Chamoun M, Bouysset M. Anatomy of the foot and ankle. Bouysset M, editors. Bone and Joint Disorders of the Foot and Ankle Berlin, Heielberg: Springer. (1998). 10.1007/978-3-662-06132-9_1 - DOI
-
- Bava E, Charlton T, Thordarson D. Ankle fracture syndesmosis fixation and management: the current practice of orthopedic surgeons. Am J Orthop (Belle Mead NJ). (2010) 39:242–6. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

