A Review of the Presentation of Overdiagnosis in Cancer Screening Patient Decision Aids
- PMID: 35187246
- PMCID: PMC8855414
- DOI: 10.1177/2381468319881447
A Review of the Presentation of Overdiagnosis in Cancer Screening Patient Decision Aids
Abstract
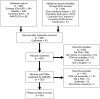
Introduction. Patient decision aid (PDA) certification standards recommend including the positive and negative features of each option of the decision. This review describes the inclusion of concepts related to overdiagnosis and overtreatment, negative features often ambiguously defined, in cancer screening PDAs. Methods. Our process followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We reviewed 1) current systematic reviews of decision aids, 2) the Ottawa Hospital Research Institute Decision Aid Library Inventory, and 3) a web-based, gray literature search. Two independent reviewers identified and evaluated PDAs using content analysis. Reviewers coded whether overdiagnosis/overtreatment was described as 1) detecting cancer that would not lead to death, 2) detecting cancer that would not cause symptoms, and/or 3) a potential harm or consequence of screening. Coding discrepancies were resolved through consensus. Results. A total of 904 records (e.g., articles, PDAs) were reviewed and 85 PDAs were identified: prostate (n = 36), breast (n = 26), lung (n = 10), colorectal (n = 10), and other (n = 3). Sixty-seven PDAs included concepts related to overdiagnosis/overtreatment; 57 (67.1%) used a term other than overdiagnosis/overtreatment, 23 (27.1%) used the specific term "overdiagnosis," and 13 (15.3%) used "overtreatment." PDAs described overdiagnosis/overtreatment as a potential harm or consequence of screening (n = 62) and/or a detection of a cancer that would not cause symptoms (n = 49). Thirty-six described overdiagnosis as the detection of a cancer that would not result in death. Twenty PDAs described the probabilities associated with overdiagnosis/overtreatment. Conclusions. Over three quarters of cancer screening PDAs addressed concepts related to overdiagnosis/overtreatment, yet terminology was inconsistent and few included probability estimates. Consistent terminology and minimum standards to describe overdiagnosis/overtreatment would help guide the design and certification of cancer screening PDAs.
Keywords: cancer; decision aids; health communication; screening; systematic review.
© The Author(s) 2019.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Woolf SH, Chan EY, Harris R, et al.. Promoting informed choice: transforming health care to dispense knowledge for decision making. Ann Intern Med. 2005;143(4):293–300. - PubMed
-
- O’Connor AM, Bennett C, Stacey D, et al.. Do patient decision aids meet effectiveness criteria of the International Patient Decision Aid Standards Collaboration? A systematic review and meta-analysis. Med Decis Making. 2007;27(5):554–74. - PubMed
-
- Poddar U, Brownlee S, Stacey D, Volk RJ, Williams JW, Elwyn G. Patient decision aids: a case for certification at the national level in the United States. J Clin Ethics. 2015;26(4):306–11. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources