Hypoxia-preconditioning of human adipose-derived stem cells enhances cellular proliferation and angiogenesis: A systematic review
- PMID: 35187291
- PMCID: PMC8848748
Hypoxia-preconditioning of human adipose-derived stem cells enhances cellular proliferation and angiogenesis: A systematic review
Abstract
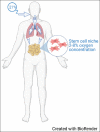
Background: Human adipose-derived stem cells (hADSCs) have gained attention lately because of their ease of harvesting and ability to be substantially multiplied in laboratory cultures. Stem cells are usually cultured under atmospheric conditions; however, preconditioning stem cells under hypoxic conditions seems beneficial.
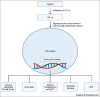
Aim: This systematic review aims to investigate the effect of hypoxia preconditioning and its impact on the proliferation and angiogenic capacity of the hADSCs.
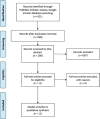
Methods: We performed a systematic review by searching PubMed, Scopus, Embase, and Google Scholar databases from all years through March 22, 2021, following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Medical Subject Headings terms "adipose-derived stem cell," "Hypoxia," "cell proliferation," and "angiogenesis" guided our search. Only articles written in English using experimental models comparing a preconditioned group against a control group of hADSCs with data on proliferation and angiogenic capacity were included.
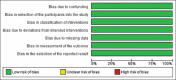
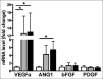
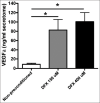
Results: Our search yielded a total of 321 articles. 11 articles met our inclusion criteria and were ultimately included in this review. Two studies induced hypoxia using hypoxia-inducible factor-1 alpha stabilizing agents, while nine reached hypoxia by changing oxygen tension conditions around the cells. Four articles conducted in-vivo studies to correlate their in-vitro findings, which proved to be consistent. Although 1 article indicated cell proliferation inhibition with hypoxia preconditioning, the remaining 10 found enhanced proliferation in preconditioned groups compared to controls. All articles showed an enhanced angiogenic capacity of hADSCs after hypoxia preconditioning.
Conclusion: In this review, we found evidence to support hypoxia preconditioning of hADSCs before implantation. Benefits include enhanced cell proliferation with a faster population doubling rate and increased secretion of multiple angiogenic growth factors, enhancing angiogenesis capacity.
Relevance for patients: Although regenerative therapy is a promising field of study and treatment in medicine, much is still unknown. The potential for angiogenic therapeutics with stem cells is high, but more so, if we discover ways to enhance their natural angiogenic properties. Procedures and pathologies alike require the assistance of angiogenic treatments to improve outcome, such is the case with skin grafts, muscle flaps, skin flaps, or myocardial infarction to mention a few. Enhanced angiogenic properties of stem cells may pave the way for better outcomes and results for patients.
Keywords: adipose-derived stem cells; angiogenesis; cell hypoxia; cell proliferation; growth factors; human stem cells; regenerative medicine.
Copyright: © Whioce Publishing Pte. Ltd.
Conflict of interest statement
The authors declare no conflict of interest
Figures


References
-
- Caplan AI. Mesenchymal stem cells in regenerative medicine. In: Atala A, Lanza R, Mikos AG, Nerem R, editors. Principles of Regenerative Medicine. 3rd ed. Boston: Academic Press; 2019. pp. 219–27. Ch. 15.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
