ATP Synthase K+- and H+-fluxes Drive ATP Synthesis and Enable Mitochondrial K+-"Uniporter" Function: II. Ion and ATP Synthase Flux Regulation
- PMID: 35187492
- PMCID: PMC8850977
- DOI: 10.1093/function/zqac001
ATP Synthase K+- and H+-fluxes Drive ATP Synthesis and Enable Mitochondrial K+-"Uniporter" Function: II. Ion and ATP Synthase Flux Regulation
Abstract
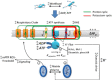
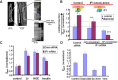
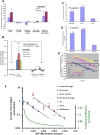
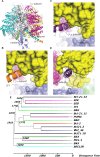
We demonstrated that ATP synthase serves the functions of a primary mitochondrial K+ "uniporter," i.e., the primary way for K+ to enter mitochondria. This K+ entry is proportional to ATP synthesis, regulating matrix volume and energy supply-vs-demand matching. We show that ATP synthase can be upregulated by endogenous survival-related proteins via IF1. We identified a conserved BH3-like domain of IF1 which overlaps its "minimal inhibitory domain" that binds to the β-subunit of F1. Bcl-xL and Mcl-1 possess a BH3-binding-groove that can engage IF1 and exert effects, requiring this interaction, comparable to diazoxide to augment ATP synthase's H+ and K+ flux and ATP synthesis. Bcl-xL and Mcl-1, but not Bcl-2, serve as endogenous regulatory ligands of ATP synthase via interaction with IF1 at this BH3-like domain, to increase its chemo-mechanical efficiency, enabling its function as the recruitable mitochondrial KATP-channel that can limit ischemia-reperfusion injury. Using Bayesian phylogenetic analysis to examine potential bacterial IF1-progenitors, we found that IF1 is likely an ancient (∼2 Gya) Bcl-family member that evolved from primordial bacteria resident in eukaryotes, corresponding to their putative emergence as symbiotic mitochondria, and functioning to prevent their parasitic ATP consumption inside the host cell.
Keywords: ATP synthase regulation; ATPase Inhibitory Factor-1 (IF₁); Bcl-2 family proteins; mitochondrial permeability transition pore; mitochondrial potassium transport; volume regulation.
Published by Oxford University Press on behalf of American Physiological Society 2022.
Figures



Comment in
-
Rethinking Mitchell's Chemiosmotic Theory: Potassium Dominates Over Proton Flux to Drive Mitochondrial F1Fo-ATP Synthase.Function (Oxf). 2022 Mar 9;3(2):zqac012. doi: 10.1093/function/zqac012. eCollection 2022. Function (Oxf). 2022. PMID: 35399493 Free PMC article. No abstract available.
-
Setting the Record Straight: A New Twist on the Chemiosmotic Mechanism of Oxidative Phosphorylation.Function (Oxf). 2022 Apr 19;3(3):zqac018. doi: 10.1093/function/zqac018. eCollection 2022. Function (Oxf). 2022. PMID: 35601666 Free PMC article. No abstract available.
References
-
- Garlid KD, Dos Santos P, Xie ZJ, Costa AD, Paucek P. Mitochondrial potassium transport: the role of the mitochondrial ATP-sensitive K(+) channel in cardiac function and cardioprotection. Biochim Biophys Acta. 2003;1606(1-3):1–21. - PubMed
-
- Juhaszova M, Wang S, Zorov DBet al. . The identity and regulation of the mitochondrial permeability transition pore: where the known meets the unknown. Ann NY Acad Sci. 2008;1123(1):197–212.. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop.... - PubMed
-
- Juhaszova M, Zorov DB, Kim SHet al. . Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113(11):1535–1549.. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop.... - PMC - PubMed
-
- Zorov DB, Juhaszova M, Yaniv Y, Nuss HB, Wang S, Sollott SJ.. Regulation and pharmacology of the mitochondrial permeability transition pore. Cardiovasc Res. 2009;83(2):213–225.. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop.... - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
