Impaired detection of omicron by SARS-CoV-2 rapid antigen tests
- PMID: 35187580
- PMCID: PMC8858605
- DOI: 10.1007/s00430-022-00730-z
Impaired detection of omicron by SARS-CoV-2 rapid antigen tests
Abstract
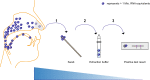
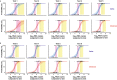
Since autumn 2020, rapid antigen tests (RATs) have been implemented in several countries as an important pillar of the national testing strategy to rapidly screen for infections on site during the SARS-CoV-2 pandemic. The current surge in infection rates around the globe is driven by the variant of concern (VoC) omicron (B.1.1.529). Here, we evaluated the performance of nine SARS-CoV-2 RATs in a single-centre laboratory study. We examined a total of 115 SARS-CoV-2 PCR-negative and 166 SARS-CoV-2 PCR-positive respiratory swab samples (101 omicron, 65 delta (B.1.617.2)) collected from October 2021 until January 2022 as well as cell culture-expanded clinical isolates of both VoCs. In an assessment of the analytical sensitivity in clinical specimen, the 50% limit of detection (LoD50) ranged from 1.77 × 106 to 7.03 × 107 RNA copies subjected to the RAT for omicron compared to 1.32 × 105 to 2.05 × 106 for delta. To score positive in these point-of-care tests, up to 10-fold (LoD50) or 101-fold (LoD95) higher virus loads were required for omicron- compared to delta-containing samples. The rates of true positive test results for omicron samples in the highest virus load category (Ct values < 25) ranged between 31.4 and 77.8%, while they dropped to 0-8.3% for samples with intermediate Ct values (25-30). Of note, testing of expanded virus stocks suggested a comparable RAT sensitivity of both VoCs, questioning the predictive value of this type of in vitro-studies for clinical performance. Given their importance for national test strategies in the current omicron wave, awareness must be increased for the reduced detection rate of omicron infections by RATs and a short list of suitable RATs that fulfill the minimal requirements of performance should be rapidly disclosed.
Keywords: Diagnostic test; Lateral flow; Nucleocapsid protein; SARS-CoV-2 rapid antigen test; Sensitivity; Specificity; VoC.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

