Clonazepam monotherapy for treating people with newly diagnosed epilepsy
- PMID: 35187637
- PMCID: PMC8859772
- DOI: 10.1002/14651858.CD013028.pub3
Clonazepam monotherapy for treating people with newly diagnosed epilepsy
Abstract
Background: Epilepsy is one of the most common neurological disorders worldwide, with an age-adjusted prevalence of 4 to 8 per 1000 population and an age-adjusted incidence of 44 per 100,000 person-years in developed countries. Monotherapy represents the best therapeutic option in people with newly diagnosed epilepsy. This is an updated version of the original Cochrane Review published in 2019, Issue 11.
Objectives: To assess the efficacy and tolerability of oral clonazepam used as monotherapy for newly diagnosed epilepsy, compared with placebo or a different anti-seizure medication.
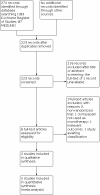
Search methods: For the latest update of this review we searched the following databases on 14 September 2021: the Cochrane Register of Studies (CRS Web) and MEDLINE (Ovid) (1946 to 13 September 2021). CRS Web includes randomized controlled trials (RCTs) or quasi-RCTs from PubMed, Embase, ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform (ICTRP), the Cochrane Central Register of Controlled Trials (CENTRAL), and the Specialized Registers of Cochrane Review Groups, including Epilepsy.
Selection criteria: We included RCTs or quasi-RCTs comparing oral clonazepam used as monotherapy treatment versus placebo or a different anti-seizure medication (active comparator) in people of any age with newly diagnosed epilepsy, defined according to the clinical practical definition proposed by the International League Against Epilepsy (ILAE).
Data collection and analysis: The following outcomes were considered: proportion of participants seizure-free at one, three, six, 12, and 24 months after randomization; proportion of responders (those with at least a 50% reduction in seizure frequency from baseline to end of treatment); proportion of participants with treatment-emergent adverse events (TEAEs) during the treatment period or leading to discontinuation during the treatment period; proportion of dropouts/withdrawals due to side effects, lack of efficacy or other reasons; and improvement in quality of life, as assessed by validated and reliable rating scales. Two review authors independently screened all titles and abstracts to assess the eligibility of publications identified by the searches. We independently extracted data from trial reports and cross-checked them for accuracy. Any disagreements between the two authors regarding data extraction were resolved by discussion and consensus. We scrutinized trials and evaluated the methodological quality of all included studies. We used GRADE assessment criteria to evaluate the certainty of the evidence.
Main results: Two randomized controlled trials had already been included in the previous version of the review, with a total of 115 participants. One study compared clonazepam to carbamazepine as monotherapy for participants with newly diagnosed psychomotor epilepsy (a condition corresponding to what is now termed mesial temporal lobe epilepsy). One study (published as an abstract) compared clonazepam to ethosuximide as monotherapy for children with absence seizures. Based on the available data and the details on methodology provided, we judged both studies as being at unclear or high risk of bias for the domains assessed (apart from the selective reporting (reporting bias) domain - we judged one study as being at low risk of bias and the other study at high risk of bias). In the study comparing clonazepam to carbamazepine, no difference was found between the groups regarding the proportion of participants who were seizure-free at one month after randomization (risk ratio (RR) 1.97, 95% confidence interval (CI) 0.99 to 3.94; 30 participants; very low-certainty evidence), three months after randomization (RR 1.19, 95% CI 0.62 to 2.29; 26 participants; very low-certainty evidence), and six months after randomization (RR 0.50, 95% CI 0.09 to 2.73; 9 participants; very low-certainty evidence). No statistical difference was found between clonazepam and carbamazepine in terms of proportion of participants with TEAEs leading to discontinuation (RR 2.61, 95% CI 0.80 to 8.52; 36 participants; very low-certainty evidence) and in terms of dropouts/withdrawals due to side effects, lack of efficacy or other reasons (RR 1.56, 95% CI 0.61 to 4.02; 36 participants; very low-certainty evidence). The study did not provide any information on our other prespecified outcomes of interest. The study comparing clonazepam to ethosuximide did not provide any data on efficacy. The proportion of dropouts/withdrawal was higher in the group receiving clonazepam compared to the group receiving ethosuximide (RR 3.63, 95% CI 1.12 to 11.74; 79 participants; very low-certainty evidence). No information on other outcomes of interest was provided in this study.
Authors' conclusions: We did not find any new studies since the last version of this review. There is only limited and very low-certainty evidence from randomized controlled trials on the efficacy and tolerability of clonazepam used in monotherapy for the treatment of epilepsy. No difference in efficacy and tolerability was found in a small trial comparing clonazepam to carbamazepine for the treatment of mesial temporal lobe epilepsy. Clonazepam was less well tolerated than ethosuximide in a trial of children with absence seizures, however no comparative data on efficacy were provided. There is currently insufficient evidence to support the use of clonazepam as monotherapy treatment for epilepsy.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
FB: none known. SCI: none known. NLB: none known. SL: none known.
Figures





Update of
-
Clonazepam monotherapy for treating people with newly diagnosed epilepsy.Cochrane Database Syst Rev. 2019 Nov 19;2019(11):CD013028. doi: 10.1002/14651858.CD013028.pub2. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2022 Feb 21;2:CD013028. doi: 10.1002/14651858.CD013028.pub3. PMID: 31742671 Free PMC article. Updated.
Similar articles
-
Treatments for seizures in catamenial (menstrual-related) epilepsy.Cochrane Database Syst Rev. 2021 Sep 16;9(9):CD013225. doi: 10.1002/14651858.CD013225.pub3. Cochrane Database Syst Rev. 2021. PMID: 34528245 Free PMC article.
-
Clonazepam monotherapy for treating people with newly diagnosed epilepsy.Cochrane Database Syst Rev. 2019 Nov 19;2019(11):CD013028. doi: 10.1002/14651858.CD013028.pub2. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2022 Feb 21;2:CD013028. doi: 10.1002/14651858.CD013028.pub3. PMID: 31742671 Free PMC article. Updated.
-
Pregabalin add-on for drug-resistant focal epilepsy.Cochrane Database Syst Rev. 2022 Mar 29;3(3):CD005612. doi: 10.1002/14651858.CD005612.pub5. Cochrane Database Syst Rev. 2022. PMID: 35349176 Free PMC article.
-
Brivaracetam add-on therapy for drug-resistant epilepsy.Cochrane Database Syst Rev. 2022 Mar 14;3(3):CD011501. doi: 10.1002/14651858.CD011501.pub3. Cochrane Database Syst Rev. 2022. PMID: 35285519 Free PMC article.
-
Stimulant and non-stimulant drug therapy for people with attention deficit hyperactivity disorder and epilepsy.Cochrane Database Syst Rev. 2022 Jul 13;7(7):CD013136. doi: 10.1002/14651858.CD013136.pub2. Cochrane Database Syst Rev. 2022. PMID: 35844168 Free PMC article.
Cited by
-
Established and emerging GABAA receptor pharmacotherapy for epilepsy.Front Pharmacol. 2024 Feb 21;15:1341472. doi: 10.3389/fphar.2024.1341472. eCollection 2024. Front Pharmacol. 2024. PMID: 38449810 Free PMC article. Review.
-
The potential of dibenzazepine carboxamides in cancer therapy.Front Pharmacol. 2025 Mar 28;16:1564911. doi: 10.3389/fphar.2025.1564911. eCollection 2025. Front Pharmacol. 2025. PMID: 40223925 Free PMC article. Review.
-
NLRP3 Inflammasome Inhibitors for Antiepileptogenic Drug Discovery and Development.Int J Mol Sci. 2024 May 31;25(11):6078. doi: 10.3390/ijms25116078. Int J Mol Sci. 2024. PMID: 38892264 Free PMC article. Review.
References
References to studies included in this review
Mikkelsen 1981 {published data only}
-
- Mikkelsen B, Berggreen P, Joensen P, Kristensen O, Kohler O, Mikkelsen BO.Clonazepam (Rivotril) and carbamazepine (Tegretol) in psychomotor epilepsy: a randomized multicenter trial. Epilepsia 1981;22(4):415-20. [PMID: ] - PubMed
-
- Mikkelsen B, Berggreen P, Joensen P, Kristensen O, Kohler O, Mikkelsen BO.Treatment of psychomotor epilepsy with clonazepam (Rivotril®) and carbamazepine (Tegretol®): a randomized multicentre trial. Acta Neurologica Scandinavica 1980;62(S78):214-5. [DOI: 10.1111/j.1600-0404.1980.tb05441.x] - DOI
Sato 1977 {published data only}
-
- Sato S, Penry JK, Dreifuss FE, Dyken PR.Clonazepam in the treatment of absence seizures: a double-blind clinical trial. Neurology 1977;27(4):371, Abstract no: CN 20.
References to studies excluded from this review
Dahlin 2000 {published data only}
-
- Dahlin M, Knutsson E, Amark P, Nergardh A.Reduction of epileptiform activity in response to low-dose clonazepam in children with epilepsy: a randomized double-blind study. Epilepsia 2000;41(3):308-15. [PMID: ] - PubMed
Mitsudome 1997 {published data only}
-
- Mitsudome A, Ohfu M, Yasumoto S, Ogawa A, Hirose S, Ogata H, et al.The effectiveness of clonazepam on the Rolandic discharges. Brain & Development 1997;19(4):274-8. [PMID: ] - PubMed
Yamatogi 1997 {published data only}
-
- Yamatogi Y, Ohtahara S, Shigematsu H, Oguni H, Nakashima M.Single blind comparative study of clobazam (NH-15) with clonazepam in intractable childhood epilepsies. Journal of the Japan Epilepsy Society 1997;15(2):110-21. [DOI: 10.3805/jjes.15.110] - DOI
References to studies awaiting assessment
Miyasaka 1977 {published data only}
-
- Miyasaka M, Hyugano H, Fukushima Y.Efficacy of clonazepam compared with nitrazepam by double blind cross-over trial on psychomotor epilepsy. Sinsyoseishinigaku 1977;6(9):1267-86.
Additional references
Browne 1976
-
- Browne TR.Clonazepam: a review of a new anticonvulsant drug. Archives of Neurology 1976;33(5):326-32. [PMID: ] - PubMed
Browne 1978
-
- Browne TR.Clonazepam. New England Journal of Medicine 1978;299(15):812-6. [PMID: ] - PubMed
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A.Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [PMID: ] - PubMed
Fisher 2005
-
- Fisher RS, Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al.Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005;46(4):470-2. [PMID: ] - PubMed
Fisher 2014
-
- Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al.ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014;55(4):475-82. [PMID: ] - PubMed
GRADEpro GDT 2015 [Computer program]
-
- GRADEpro GDT.Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Hauser 1991
-
- Hauser WA, Annegers JF, Kurland LT.Prevalence of epilepsy in Rochester, Minnesota: 1940-1980. Epilepsia 1991;32(4):429-45. [PMID: ] - PubMed
Hauser 1993
-
- Hauser WA, Annegers JF, Kurland LT.Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935-1984. Epilepsia 1993;34(3):453-68. [PMID: ] - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S, editor(s).Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Ishikawa 1985
-
- Ishikawa A, Sakuma N, Nagashima T, Kohsaka S, Kajii N.Clonazepam monotherapy for epilepsy in childhood. Brain and Development 1985;7(6):610-3. [PMID: ] - PubMed
Keränen 1983
-
- Keränen T, Sivenius J.Side effects of carbamazepine, valproate and clonazepam during long-term treatment of epilepsy. Acta Neurologica Scandinavica. Supplementum 1983;97:69-80. [PMID: ] - PubMed
Lefebvre 2021
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al.Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Liberati 2009
Livanainen 1982
-
- Livanainen M, Himberg JJ.Valproate and clonazepam in the treatment of severe progressive myoclonus epilepsy. Archives of Neurology 1982;39(4):236-8. [PMID: ] - PubMed
Obeso 1995‐1996
-
- Obeso JA.Therapy of myoclonus. Clinical Neuroscience 1995-1996;3(4):253-7. [PMID: ] - PubMed
Perucca 2011
-
- Perucca E, Tomson T.The pharmacological treatment of epilepsy in adults. Lancet Neurology 2011;10(5):446-56. [PMID: ] - PubMed
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5).Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Sander 1996
Scheffer 2016
-
- Scheffer IE, French J, Hirsch E, Jain S, Mathern GW, Moshe SL, et al.Classification of the epilepsies: new concepts for discussion and debate - special report of the ILAE Classification Task Force of the Commission for Classification and Terminology. Epilepsia Open 2016;1(1-2):37-44. [PMID: ] - PMC - PubMed
Schunemann 2011
-
- Schunemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al.Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Trinka 2016
-
- Trinka E, Brigo F.Benzodiazepines used in the treatment of epilepsy. In: Shorvon S, Perucca E, Engel J, editors(s). The Treatment of Epilepsy. 4 edition. John Wiley & Sons, Ltd, 2016:398-417.
Wheless 2005
-
- Wheless JW, Clarke DF, Carperter D.Treatment of pediatric epilepsy: expert opinion 2005. Journal of Child Neurology 2005;20(Suppl 1):S1-S56. [PMID: ] - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

