Melanoma-derived extracellular vesicles mediate lymphatic remodelling and impair tumour immunity in draining lymph nodes
- PMID: 35188342
- PMCID: PMC8859913
- DOI: 10.1002/jev2.12197
Melanoma-derived extracellular vesicles mediate lymphatic remodelling and impair tumour immunity in draining lymph nodes
Abstract
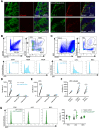
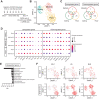
Tumour-draining lymph nodes (LNs) undergo massive remodelling including expansion of the lymphatic sinuses, a process that has been linked to lymphatic metastasis by creation of a pre-metastatic niche. However, the signals leading to these changes have not been completely understood. Here, we found that extracellular vesicles (EVs) derived from melanoma cells are rapidly transported by lymphatic vessels to draining LNs, where they selectively interact with lymphatic endothelial cells (LECs) as well as medullary sinus macrophages. Interestingly, uptake of melanoma EVs by LN-resident LECs was partly dependent on lymphatic VCAM-1 expression, and induced transcriptional changes as well as proliferation of those cells. Furthermore, melanoma EVs shuttled tumour antigens to LN LECs for cross-presentation on MHC-I, resulting in apoptosis induction in antigen-specific CD8+ T cells. In conclusion, our data identify EV-mediated melanoma-LN LEC communication as a new pathway involved in tumour progression and tumour immune inhibition, suggesting that EV uptake or effector mechanisms in LECs might represent a new target for melanoma therapy.
Keywords: exosome; immunotherapy; lymphangiogenesis; pre-metastatic niche; sentinel lymph node.
© 2022 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Arasa, J. , Collado‐Diaz, V. , Kritikos, I. , Medina‐Sanchez, J. D. , Friess, M. C. , Sigmund, E. C. , Schineis, P. , Hunter, M. C. , Tacconi, C. , Paterson, N. , Nagasawa, T. , Kiefer, F. , Makinen, T. , Detmar, M. , Moser, M. , Lämmermann, T. , & Halin, C. (2021). Upregulation of VCAM‐1 in lymphatic collectors supports dendritic cell entry and rapid migration to lymph nodes in inflammation. Journal of Experimental Medicine; 218(7). - PMC - PubMed
-
- Bald, T. , Landsberg, J. , Lopez‐Ramos, D. , Renn, M. , Glodde, N. , Jansen, P. , Gaffal, E. , Steitz, J. , Tolba, R. , Kalinke, U. , Limmer, A. , Jönsson, G. , Hölzel, M. , & Tüting, T. (2014). Immune cell–Poor melanomas benefit from PD‐1 blockade after targeted type I IFN activation. Cancer Discovery; 4(6), 674–687. - PubMed
-
- Becht, E. , McInnes, L. , Healy, J. , Dutertre, C. A. , Kwok, I. W. H. , & Ng, L. G. et al. (2018) Dimensionality reduction for visualizing single‐cell data using UMAP. Nature Biotechnology. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

