P2Y1 purinergic receptor identified as a diabetes target in a small-molecule screen to reverse circadian β-cell failure
- PMID: 35188462
- PMCID: PMC8860442
- DOI: 10.7554/eLife.75132
P2Y1 purinergic receptor identified as a diabetes target in a small-molecule screen to reverse circadian β-cell failure
Abstract
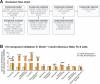
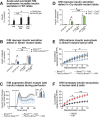
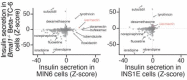
The mammalian circadian clock drives daily oscillations in physiology and behavior through an autoregulatory transcription feedback loop present in central and peripheral cells. Ablation of the core clock within the endocrine pancreas of adult animals impairs the transcription and splicing of genes involved in hormone exocytosis and causes hypoinsulinemic diabetes. Here, we developed a genetically sensitized small-molecule screen to identify druggable proteins and mechanistic pathways involved in circadian β-cell failure. Our approach was to generate β-cells expressing a nanoluciferase reporter within the proinsulin polypeptide to screen 2640 pharmacologically active compounds and identify insulinotropic molecules that bypass the secretory defect in CRISPR-Cas9-targeted clock mutant β-cells. We validated hit compounds in primary mouse islets and identified known modulators of ligand-gated ion channels and G-protein-coupled receptors, including the antihelmintic ivermectin. Single-cell electrophysiology in circadian mutant mouse and human cadaveric islets revealed ivermectin as a glucose-dependent secretagogue. Genetic, genomic, and pharmacological analyses established the P2Y1 receptor as a clock-controlled mediator of the insulinotropic activity of ivermectin. These findings identify the P2Y1 purinergic receptor as a diabetes target based upon a genetically sensitized phenotypic screen.
Keywords: cell biology; circadian clock; diabetes; high-throughput screen; human; insulin; ivermectin; mouse; purinergic receptor.
Plain language summary
Circadian rhythms – ‘inbuilt’ 24-hour cycles – control many aspects of behaviour and physiology. In mammals, they operate in nearly all tissues, including those involved in glucose metabolism. Recent studies have shown that mice with faulty genes involved in circadian rhythms, the core clock genes, can develop diabetes. Diabetes arises when the body struggles to regulate blood sugar levels. In healthy individuals, the hormone insulin produced by beta cells in the pancreas regulates the amount of sugar in the blood. But when beta cells are faulty and do not generate sufficient insulin levels, or when insulin lacks the ability to stimulate cells to take up glucose, diabetes can develop. Marcheva, Weidemann, Taguchi et al. wanted to find out if diabetes caused by impaired clock genes could be treated by targeting pathways regulating the secretion of insulin. To do so, they tested over 2,500 potential drugs on genetically modified beta cells with faulty core clock genes. They further screened the drugs on mice with the same defect in their beta cells. Marcheva et al. identified one potential compound, the anti-parasite drug ivermectin, which was able to restore the secretion of insulin. When ivermectin was applied to both healthy mice and mice with faulty beta cells, the drug improved the control over glucose levels by activating a specific protein receptor that senses molecules important for storing and utilizing energy. The findings reveal new drug targets for treating forms of diabetes associated with deregulation of the pancreatic circadian clock. The drug screening strategy used in the study may also be applied to reveal mechanisms underlying other conditions associated with disrupted circadian clocks, including sleep loss and jetlag.
© 2022, Marcheva et al.
Conflict of interest statement
BM, BW, AT, KR, MN, YK, CO, JM, HL, PM, JB No competing interests declared, MP Mark Perelis is affiliated with Ionis Pharmaceuticals, Inc The author has no financial interests to declare
Figures







Similar articles
-
Induction of Core Circadian Clock Transcription Factor Bmal1 Enhances β-Cell Function and Protects Against Obesity-Induced Glucose Intolerance.Diabetes. 2021 Jan;70(1):143-154. doi: 10.2337/db20-0192. Epub 2020 Oct 21. Diabetes. 2021. PMID: 33087455 Free PMC article.
-
Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes.Nature. 2010 Jul 29;466(7306):627-31. doi: 10.1038/nature09253. Nature. 2010. PMID: 20562852 Free PMC article.
-
A role for alternative splicing in circadian control of exocytosis and glucose homeostasis.Genes Dev. 2020 Aug 1;34(15-16):1089-1105. doi: 10.1101/gad.338178.120. Epub 2020 Jul 2. Genes Dev. 2020. PMID: 32616519 Free PMC article.
-
Role of the clock gene Rev-erbα in metabolism and in the endocrine pancreas.Diabetes Obes Metab. 2015 Sep;17 Suppl 1:106-14. doi: 10.1111/dom.12522. Diabetes Obes Metab. 2015. PMID: 26332975 Review.
-
The molecular clock as a metabolic rheostat.Diabetes Obes Metab. 2015 Sep;17 Suppl 1(0 1):99-105. doi: 10.1111/dom.12521. Diabetes Obes Metab. 2015. PMID: 26332974 Free PMC article. Review.
Cited by
-
Drug repurposing of ivermectin abrogates neutrophil extracellular traps and prevents melanoma metastasis.Front Oncol. 2022 Sep 5;12:989167. doi: 10.3389/fonc.2022.989167. eCollection 2022. Front Oncol. 2022. PMID: 36132145 Free PMC article.
-
Type I Diabetes Pathoetiology and Pathophysiology: Roles of the Gut Microbiome, Pancreatic Cellular Interactions, and the 'Bystander' Activation of Memory CD8+ T Cells.Int J Mol Sci. 2023 Feb 7;24(4):3300. doi: 10.3390/ijms24043300. Int J Mol Sci. 2023. PMID: 36834709 Free PMC article. Review.
-
Pharmacological and mechanistic study of PS1, a Pdia4 inhibitor, in β-cell pathogenesis and diabetes in db/db mice.Cell Mol Life Sci. 2023 Mar 19;80(4):101. doi: 10.1007/s00018-022-04677-5. Cell Mol Life Sci. 2023. PMID: 36935456 Free PMC article.
-
Natural Compounds for Preventing Age-Related Diseases and Cancers.Int J Mol Sci. 2024 Jul 9;25(14):7530. doi: 10.3390/ijms25147530. Int J Mol Sci. 2024. PMID: 39062777 Free PMC article. Review.
-
Circadian clock communication during homeostasis and ageing.Nat Rev Mol Cell Biol. 2025 Apr;26(4):314-331. doi: 10.1038/s41580-024-00802-3. Epub 2025 Jan 3. Nat Rev Mol Cell Biol. 2025. PMID: 39753699 Review.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

