Does rapid sequence divergence preclude RNA structure conservation in vertebrates?
- PMID: 35188540
- PMCID: PMC8934657
- DOI: 10.1093/nar/gkac067
Does rapid sequence divergence preclude RNA structure conservation in vertebrates?
Abstract
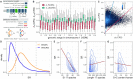
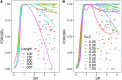
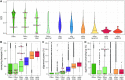
Accelerated evolution of any portion of the genome is of significant interest, potentially signaling positive selection of phenotypic traits and adaptation. Accelerated evolution remains understudied for structured RNAs, despite the fact that an RNA's structure is often key to its function. RNA structures are typically characterized by compensatory (structure-preserving) basepair changes that are unexpected given the underlying sequence variation, i.e., they have evolved through negative selection on structure. We address the question of how fast the primary sequence of an RNA can change through evolution while conserving its structure. Specifically, we consider predicted and known structures in vertebrate genomes. After careful control of false discovery rates, we obtain 13 de novo structures (and three known Rfam structures) that we predict to have rapidly evolving sequences-defined as structures where the primary sequences of human and mouse have diverged at least twice as fast (1.5 times for Rfam) as nearby neutrally evolving sequences. Two of the three known structures function in translation inhibition related to infection and immune response. We conclude that rapid sequence divergence does not preclude RNA structure conservation in vertebrates, although these events are relatively rare.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
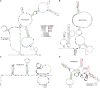
References
-
- Yao Z., Weinberg Z., Ruzzo W.. CMfinder–a covariance model based RNA motif finding algorithm. Bioinformatics. 2006; 22:445–452. - PubMed
-
- Washietl S., Hofacker I., Lukasser M., Huttenhofer A., Stadler P.. Mapping of conserved RNA secondary structures predicts thousands of functional noncoding RNAs in the human genome. Nat. Biotechnol. 2005; 23:1383–1390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

