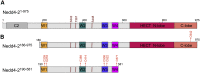
Nedd4-2 binding to 14-3-3 modulates the accessibility of its catalytic site and WW domains
- PMID: 35189105
- PMCID: PMC9034186
- DOI: 10.1016/j.bpj.2022.02.025
Nedd4-2 binding to 14-3-3 modulates the accessibility of its catalytic site and WW domains
Abstract
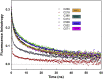
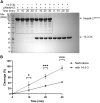
Neural precursor cells expressed developmentally downregulated protein 4-2 (Nedd4-2), a homologous to the E6-AP carboxyl terminus (HECT) ubiquitin ligase, triggers the endocytosis and degradation of its downstream target molecules by regulating signal transduction through interactions with other targets, including 14-3-3 proteins. In our previous study, we found that 14-3-3 binding induces a structural rearrangement of Nedd4-2 by inhibiting interactions between its structured domains. Here, we used time-resolved fluorescence intensity and anisotropy decay measurements, together with fluorescence quenching and mass spectrometry, to further characterize interactions between Nedd4-2 and 14-3-3 proteins. The results showed that 14-3-3 binding affects the emission properties of AEDANS-labeled WW3, WW4, and, to a lesser extent, WW2 domains, and reduces their mobility, but not those of the WW1 domain, which remains mobile. In contrast, 14-3-3 binding has the opposite effect on the active site of the HECT domain, which is more solvent exposed and mobile in the complexed form than in the apo form of Nedd4-2. Overall, our results suggest that steric hindrance of the WW3 and WW4 domains combined with conformational changes in the catalytic domain may account for the 14-3-3 binding-mediated regulation of Nedd4-2.
Copyright © 2022 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




Comment in
-
Challenges of studying 14-3-3 protein-protein interactions with full-length protein partners.Biophys J. 2022 Apr 5;121(7):1115-1116. doi: 10.1016/j.bpj.2022.03.007. Epub 2022 Mar 9. Biophys J. 2022. PMID: 35320703 Free PMC article. No abstract available.
Similar articles
-
The role of Nedd4-1 WW domains in binding and regulating human organic anion transporter 1.Am J Physiol Renal Physiol. 2016 Aug 1;311(2):F320-9. doi: 10.1152/ajprenal.00153.2016. Epub 2016 May 25. Am J Physiol Renal Physiol. 2016. PMID: 27226107 Free PMC article.
-
Affinity and specificity of interactions between Nedd4 isoforms and the epithelial Na+ channel.J Biol Chem. 2003 May 30;278(22):20019-28. doi: 10.1074/jbc.M211153200. Epub 2003 Mar 22. J Biol Chem. 2003. PMID: 12654927
-
Lysine 63-linked polyubiquitination of the dopamine transporter requires WW3 and WW4 domains of Nedd4-2 and UBE2D ubiquitin-conjugating enzymes.J Biol Chem. 2010 Mar 5;285(10):7645-56. doi: 10.1074/jbc.M109.058990. Epub 2010 Jan 5. J Biol Chem. 2010. PMID: 20051513 Free PMC article.
-
Nedd4 and Nedd4-2: closely related ubiquitin-protein ligases with distinct physiological functions.Cell Death Differ. 2010 Jan;17(1):68-77. doi: 10.1038/cdd.2009.84. Cell Death Differ. 2010. PMID: 19557014 Free PMC article. Review.
-
NEDD4 E3 Ligases: Functions and Mechanisms in Bone and Tooth.Int J Mol Sci. 2022 Sep 1;23(17):9937. doi: 10.3390/ijms23179937. Int J Mol Sci. 2022. PMID: 36077334 Free PMC article. Review.
Cited by
-
Challenges of studying 14-3-3 protein-protein interactions with full-length protein partners.Biophys J. 2022 Apr 5;121(7):1115-1116. doi: 10.1016/j.bpj.2022.03.007. Epub 2022 Mar 9. Biophys J. 2022. PMID: 35320703 Free PMC article. No abstract available.
-
Deciphering the maize gene ZmGF14-3: implications for plant height based on co-expression networks.Front Plant Sci. 2024 Jul 5;15:1397058. doi: 10.3389/fpls.2024.1397058. eCollection 2024. Front Plant Sci. 2024. PMID: 39036353 Free PMC article.
-
Primate-specific isoform of Nedd4-1 regulates substrate binding via Ser/Thr phosphorylation and 14-3-3 binding.Sci Rep. 2023 Oct 20;13(1):17903. doi: 10.1038/s41598-023-44761-9. Sci Rep. 2023. PMID: 37863970 Free PMC article.
-
Structural insights into the functional roles of 14-3-3 proteins.Front Mol Biosci. 2022 Sep 16;9:1016071. doi: 10.3389/fmolb.2022.1016071. eCollection 2022. Front Mol Biosci. 2022. PMID: 36188227 Free PMC article. Review.
-
Look for the Scaffold: Multifaceted Regulation of Enzyme Activity by 14-3-3 Proteins.Physiol Res. 2024 Aug 30;73(S1):S401-S412. doi: 10.33549/physiolres.935306. Epub 2024 Apr 22. Physiol Res. 2024. PMID: 38647170 Free PMC article. Review.
References
-
- Itani O.A., Campbell J.R., et al. Thomas C.P. Alternate promoters and variable splicing lead to hNedd4-2 isoforms with a C2 domain and varying number of WW domains. Am. J. Physiol. Ren. Physiol. 2003;285:F916–F929. - PubMed
-
- Fotia A.B., Ekberg J., et al. Kumar S. Regulation of neuronal voltage-gated sodium channels by the ubiquitin-protein ligases Nedd4 and Nedd4-2. J. Biol. Chem. 2004;279:28930–28935. - PubMed
-
- Kamynina E., Debonneville C., Bens M., Vandewalle A., Staub O. A novel mouse Nedd4 protein suppresses the activity of the epithelial Na+ channel. FASEB J. 2001;15:204–214. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

