The S-layer homology domains of Paenibacillus alvei surface protein SpaA bind to cell wall polysaccharide through the terminal monosaccharide residue
- PMID: 35189140
- PMCID: PMC8942822
- DOI: 10.1016/j.jbc.2022.101745
The S-layer homology domains of Paenibacillus alvei surface protein SpaA bind to cell wall polysaccharide through the terminal monosaccharide residue
Abstract
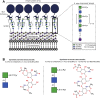
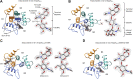
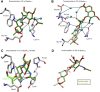
Self-assembling (glyco)protein surface layers (S-layers) are ubiquitous prokaryotic cell-surface structures involved in structural maintenance, nutrient diffusion, host adhesion, virulence, and other processes, which makes them appealing targets for therapeutics and biotechnological applications as biosensors or drug delivery systems. However, unlocking this potential requires expanding our understanding of S-layer properties, especially the details of surface-attachment. S-layers of Gram-positive bacteria often are attached through the interaction of S-layer homology (SLH) domain trimers with peptidoglycan-linked secondary cell wall polymers (SCWPs). Cocrystal structures of the SLH domain trimer from the Paenibacillus alvei S-layer protein SpaA (SpaASLH) with synthetic, terminal SCWP disaccharide and trisaccharide analogs, together with isothermal titration calorimetry binding analyses, reveal that while SpaASLH accommodates longer biologically relevant SCWP ligands within both its primary (G2) and secondary (G1) binding sites, the terminal pyruvylated ManNAc moiety serves as the nearly exclusive SCWP anchoring point. Binding is accompanied by displacement of a flexible loop adjacent to the receptor site that enhances the complementarity between protein and ligand, including electrostatic complementarity with the terminal pyruvate moiety. Remarkably, binding of the pyruvylated monosaccharide SCWP fragment alone is sufficient to cause rearrangement of the receptor-binding sites in a manner necessary to accommodate longer SCWP fragments. The observation of multiple conformations in longer oligosaccharides bound to the protein, together with the demonstrated functionality of two of the three SCWP receptor-binding sites, reveals how the SpaASLH-SCWP interaction has evolved to accommodate longer SCWP ligands and alleviate the strain inherent to bacterial S-layer adhesion during growth and division.
Keywords: S-layer; SLH domain; X-ray crystallography; cell wall anchoring; isothermal titration calorimetry; secondary cell wall polymer.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures

References
-
- Engelhardt H. Are S-layers exoskeletons? The basic function of protein surface layers revisited. J. Struct. Biol. 2007;160:115–124. - PubMed
-
- Sleytr U.B., Egelseer E.M., Ilk N., Pum D., Schuster B. S-layers as a basic building block in a molecular construction kit. FEBS J. 2007;274:323–334. - PubMed
-
- Sleytr U.B., Messner P., Pum D., Sára M. Crystalline bacterial cell surface layers. Mol. Microbiol. 1993;10:911–916. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

