Nucleosides and emerging viruses: A new story
- PMID: 35189369
- PMCID: PMC8856764
- DOI: 10.1016/j.drudis.2022.02.013
Nucleosides and emerging viruses: A new story
Abstract
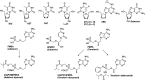
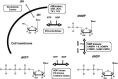
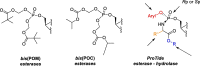
With several US Food and Drug Administration (FDA)-approved drugs and high barriers to resistance, nucleoside and nucleotide analogs remain the cornerstone of antiviral therapies for not only herpesviruses, but also HIV and hepatitis viruses (B and C); however, with the exception of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), for which vaccines have been developed at unprecedented speed, there are no vaccines or small antivirals yet available for (re)emerging viruses, which are primarily RNA viruses. Thus, herein, we present an overview of ribonucleoside analogs recently developed and acting as inhibitors of the viral RNA-dependent RNA polymerase (RdRp). They are new lead structures that will be exploited for the discovery of new antiviral nucleosides.
Keywords: (Re)emerging RNA viruses; Antiviral therapy; Broad-spectrum antiviral agents; Nucleoside analogues; RNA-dependent RNA polymerase (RdRp).
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
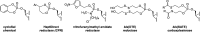

Similar articles
-
A review on the interaction of nucleoside analogues with SARS-CoV-2 RNA dependent RNA polymerase.Int J Biol Macromol. 2021 Jun 30;181:605-611. doi: 10.1016/j.ijbiomac.2021.03.112. Epub 2021 Mar 22. Int J Biol Macromol. 2021. PMID: 33766591 Free PMC article. Review.
-
Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study.Life Sci. 2020 Jul 15;253:117592. doi: 10.1016/j.lfs.2020.117592. Epub 2020 Mar 25. Life Sci. 2020. PMID: 32222463 Free PMC article.
-
RNA-dependent RNA polymerase of SARS-CoV-2 as a therapeutic target.J Med Virol. 2021 Jan;93(1):300-310. doi: 10.1002/jmv.26264. Epub 2020 Jul 19. J Med Virol. 2021. PMID: 32633831 Review.
-
Broad-Spectrum Antiviral Strategies and Nucleoside Analogues.Viruses. 2021 Apr 13;13(4):667. doi: 10.3390/v13040667. Viruses. 2021. PMID: 33924302 Free PMC article. Review.
-
Biostructural Models for the Binding of Nucleoside Analogs to SARS-CoV-2 RNA-Dependent RNA Polymerase.J Chem Inf Model. 2021 Mar 22;61(3):1402-1411. doi: 10.1021/acs.jcim.0c01277. Epub 2021 Mar 3. J Chem Inf Model. 2021. PMID: 33655751
Cited by
-
Sofosbuvir Suppresses the Genome Replication of DENV1 in Human Hepatic Huh7 Cells.Int J Mol Sci. 2024 Feb 7;25(4):2022. doi: 10.3390/ijms25042022. Int J Mol Sci. 2024. PMID: 38396699 Free PMC article.
-
Potentiality of Nucleoside as Antioxidant by Analysis on Oxidative Susceptibility, Drug Discovery, and Synthesis.Curr Med Chem. 2025;32(5):880-906. doi: 10.2174/0109298673264900231023050108. Curr Med Chem. 2025. PMID: 37933214 Review.
-
Cobalt-assisted route to rare carbocyclic C-ribonucleosides.RSC Adv. 2023 Oct 20;13(44):30777-30786. doi: 10.1039/d3ra04937j. eCollection 2023 Oct 18. RSC Adv. 2023. PMID: 37869399 Free PMC article.
-
Synthesis of Natural and Sugar-Modified Nucleosides Using the Iodine/Triethylsilane System as N-Glycosidation Promoter.Int J Mol Sci. 2024 Aug 20;25(16):9030. doi: 10.3390/ijms25169030. Int J Mol Sci. 2024. PMID: 39201716 Free PMC article.
-
Synthesis of Polycyclic Hetero-Fused 7-Deazapurine Heterocycles and Nucleosides through C-H Dibenzothiophenation and Negishi Coupling.J Am Chem Soc. 2022 Oct 26;144(42):19437-19446. doi: 10.1021/jacs.2c07517. Epub 2022 Oct 16. J Am Chem Soc. 2022. PMID: 36245092 Free PMC article.
References
-
- De Clercq E. Milestones in the discovery of antiviral agents: nucleosides and nucleotides. Acta Pharm Sin B. 2012;2:535–548.
-
- Agrofoglio L.A., Challand S.R. Kluwer Academic Publishers; Dordrecht: 1998. Acyclic, Carbocyclic and L-Nucleosides.
-
- Prusoff W.H. Synthesis and biological activities of iododeoxyuridine, an analog of thymidine. Biochim Biophys Acta. 1959;32:295–296. - PubMed
-
- Huryn D.M., Okabe M. AIDS-driven nucleoside chemistry. Chem Rev. 1992;92:1745–1768.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

