Effectiveness and Durability of COVID-19 Vaccination in 9447 Patients With IBD: A Systematic Review and Meta-Analysis
- PMID: 35189387
- PMCID: PMC8856753
- DOI: 10.1016/j.cgh.2022.02.030
Effectiveness and Durability of COVID-19 Vaccination in 9447 Patients With IBD: A Systematic Review and Meta-Analysis
Abstract
Background and aims: The serological responses after severe acute respiratory syndrome coronavirus 2 vaccination may be attenuated in immunocompromised individuals. The study aimed to systematically evaluate the seroconversion rates after complete vaccination for coronavirus disease 2019 (COVID-19) in patients with inflammatory bowel disease (IBD).
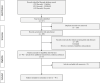
Methods: Electronic databases were searched to identify studies reporting response to COVID-19 vaccination in IBD. Pooled seroconversion rates after complete vaccination were calculated. Subgroup analysis for vaccine types was also performed. Pooled seroconversion rates for various drugs or classes were also estimated. The pooled rates of breakthrough infections in vaccinated IBD patients were estimated. The pooled neutralization rates after complete vaccination were also estimated. The studies reporting durability of titers were systematically assessed.
Results: A total of 46 studies were included. The pooled seroconversion rate for complete vaccination (31 studies, 9447 patients) was 0.96 (95% confidence interval [CI], 0.94-0.97; I2 = 90%). When compared with healthy control subjects, the pooled relative risk of seroconversion was lower (0.98; 95% CI, 0.98-0.99; I2 = 39%). The pooled seroconversion rates were statistically similar among various drug classes. The pooled positivity of neutralization assays (8 studies, 771 participants) was 0.80 (95% CI, 0.70-0.87; I2 = 82%). The pooled relative risk of breakthrough infections in vaccinated IBD patients was similar to vaccinated control subjects (0.60; 95% CI, 0.25-1.42; I2 = 79%). Most studies suggested that titers fall after 4 weeks of COVID-19 vaccination, and the decay was higher in patients on anti-tumor necrosis factor alone or combination with immunomodulators. An additional dose of COVID-19 vaccine elicited serological response in most nonresponders to complete vaccination.
Conclusions: Complete COVID-19 vaccination is associated with seroconversion in most patients with IBD. The decay in titers over time necessitates consideration of additional doses in these patients.
Keywords: Adenoviral Associated Virus; Anti-IL12/23; Anti-TNF; Crohn’s Disease; Decay; Immunization; Infliximab; Thiopurines; Ulcerative Colitis; mRNA.
Copyright © 2022 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures













References
-
- Francis A.I., Ghany S., Gilkes T., et al. Review of COVID-19 vaccine subtypes, efficacy and geographical distributions. Postgrad Med J. 2021 Aug 6 [E-pub ahead of print] - PubMed
-
- Becerril-Gaitan A., Vaca-Cartagena B.F., Ferrigno A.S., et al. Immunogenicity and risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection after coronavirus disease 2019 (COVID-19) vaccination in patients with cancer: a systematic review and meta-analysis. Eur J Cancer. 2022;160:243–260. - PMC - PubMed
Supplementary References
-
- Caldera F., Knutson K.L., Saha S., et al. Humoral immunogenicity of mRNA COVID-19 vaccines among patients with inflammatory bowel disease and healthy controls. Am J Gastroenterol. 2022;117:176–179. - PubMed
-
- Cerna K., Duricova D., Lukas M., et al. Anti-Sars-Cov-2 vaccination and antibody response in patients with inflammatory bowel disease on immune-modifying therapy. prospective single tertiary center study on 602 IBD patients. Gastroenterology. 2022;162:S101–S102. - PubMed
-
- Farkas K., Matuz M., Kata D., et al. P444 COVID-19 risk factors, infection course and vaccination among patients with inflammatory bowel disease based on a Hungarian cohort. J Crohns Colitis. 2022;16:i425.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

