Glyco-nanotechnology: A biomedical perspective
- PMID: 35189393
- PMCID: PMC11164690
- DOI: 10.1016/j.nano.2022.102542
Glyco-nanotechnology: A biomedical perspective
Abstract
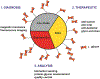
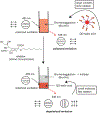
Glycans govern cellular signaling through glycan-protein and glycan-glycan crosstalk. Disruption in the crosstalk initiates 'rogue' signaling and pathology. Nanomaterials supply platforms for multivalent displays of glycans, mediate 'rogue' signal correction, and provide disease treatment modalities (therapeutics). The decorated glycans also target overexpressed lectins on unhealthy cells and direct metal nanoparticles such as gold, iron oxide, and quantum dots to the site of infection. The nanoparticles inform us about the state of the disease (diagnosis) through their distinct optical, magnetic, and electronic properties. Glyco-nanoparticles can sense disease biomarkers, report changes in protein-glycan interactions, and safeguard quality control (analysis). Here we review the current state of glyco-nanotechnology focusing on diagnosis, therapeutics, and analysis of human diseases. We highlight how glyco-nanotechnology could aid in improving diagnostic methods for the detection of disease biomarkers with magnetic resonance imaging (MRI) and fluorescence imaging (FLI), enhance therapeutics such as anti-adhesive treatment of cancer and vaccines against pneumonia, and advance analysis such as the rapid detection of pharmaceutical heparin contaminant and recombinant SARS-COV-2 spike protein. We illustrate these progressions and outline future potentials of glyco-nanotechnology in advancing human health.
Keywords: Analytical; Biomarkers; Cancer; Carbohydrates; Diagnostics; Glyco-magnetics; Glyconanotechnology; Infectious disease; Therapeutics.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


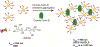

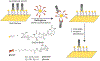












References
-
- Kelly KL, Coronado E, Zhao LL, Schatz GC. The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J Phys Chem B 2003;107(3):668–77.
-
- Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res 2016;33(10):2373–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

