Current applications and future perspective of CRISPR/Cas9 gene editing in cancer
- PMID: 35189910
- PMCID: PMC8862238
- DOI: 10.1186/s12943-022-01518-8
Current applications and future perspective of CRISPR/Cas9 gene editing in cancer
Abstract
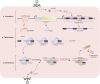
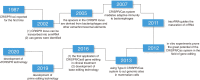
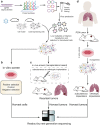
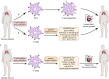
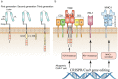
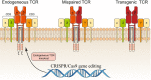
Clustered regularly interspaced short palindromic repeats (CRISPR) system provides adaptive immunity against plasmids and phages in prokaryotes. This system inspires the development of a powerful genome engineering tool, the CRISPR/CRISPR-associated nuclease 9 (CRISPR/Cas9) genome editing system. Due to its high efficiency and precision, the CRISPR/Cas9 technique has been employed to explore the functions of cancer-related genes, establish tumor-bearing animal models and probe drug targets, vastly increasing our understanding of cancer genomics. Here, we review current status of CRISPR/Cas9 gene editing technology in oncological research. We first explain the basic principles of CRISPR/Cas9 gene editing and introduce several new CRISPR-based gene editing modes. We next detail the rapid progress of CRISPR screening in revealing tumorigenesis, metastasis, and drug resistance mechanisms. In addition, we introduce CRISPR/Cas9 system delivery vectors and finally demonstrate the potential of CRISPR/Cas9 engineering to enhance the effect of adoptive T cell therapy (ACT) and reduce adverse reactions.
Keywords: CRISPR screen; CRISPR/Cas9; Gene delivery; Gene editing; Immunotherapy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they do not have any conflicts of interests.
Figures

References
-
- Labrie SJ, Samson JE, Moineau S. Bacteriophage resistance mechanisms. Nat Rev Microbiol. 2010;8:317–327. - PubMed
-
- Pourcel C, Salvignol G, Vergnaud G. CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology (Reading) 2005;151:653–663. - PubMed
-
- Bolotin A, Quinquis B, Sorokin A, Ehrlich SD. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology (Reading) 2005;151:2551–2561. - PubMed
-
- Mojica FJ, Díez-Villaseñor C, García-Martínez J, Soria E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J Mol Evol. 2005;60:174–182. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

