Can patiromer allow for intensified renin-angiotensin-aldosterone system blockade with losartan and spironolactone leading to decreased albuminuria in patients with chronic kidney disease, albuminuria and hyperkalaemia? An open-label randomised controlled trial: MorphCKD
- PMID: 35190442
- PMCID: PMC8862471
- DOI: 10.1136/bmjopen-2021-057503
Can patiromer allow for intensified renin-angiotensin-aldosterone system blockade with losartan and spironolactone leading to decreased albuminuria in patients with chronic kidney disease, albuminuria and hyperkalaemia? An open-label randomised controlled trial: MorphCKD
Abstract
Introduction: Chronic kidney disease (CKD) is associated with significantly increased morbidity and mortality. No specific treatment of the underlying condition is available for the majority of patients, but ACE-inhibitors (ACE-I) and angiotensin II-receptor blockers (ARB) slows progression in albuminuric CKD. Adding a mineralocorticoid receptor-antagonist (MRA) like spironolactone has an additive effect. However, renin-angiotensin-aldosterone system (RAAS)-blockade increases the risk of hyperkalaemia which is exacerbated by the presence of CKD. Thus, hyperkalaemia may prevent optimal use of RAAS-blockade in some patients.This project hypothesises that adding a potassium binder (patiromer) allows for improved RAAS-blockade including the use of MRA, thereby reducing albuminuria in patients with albuminuric CKD where full treatment is limited by hyperkalaemia.If successful, the study may lead to improved treatment of this subgroup of patients with CKD. Furthermore, the study will examine the feasibility of potassium binders in patients with CKD.
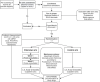
Methods and analysis: An open-label, randomised controlled trial including 140 patients with estimated glomerular filtration rate (eGFR) 25-60 mL/min/1.73 m2, a urinary albumin/creatinine ratio (UACR) >500 mg/g (or 200 mg/g if diabetes mellitus) and a current or two previous plasma-potassium >4.5 mmol/L. Patients who develop hyperkaliaemia >5.5 mmol/L during a run-in phase, in which RAAS-blockade is intesified with the possible addition of spironolactone, are randomised to 12-month treatment with maximal tolerated ACE-I/ARB and spironolactone with or without patiromer.The primary endpoint is the difference in UACR measured at randomisation and 12 months compared between the two groups. Secondary endpoints include CKD progression, episodes of hyperkalaemia, blood pressure, eGFR, markers of cardiovascular disease, diet and quality of life.
Ethics and dissemination: This study is approved by The Central Denmark Region Committees on Health Research Ethics (REFNO 1-10-72-110-20) and is registered in the EudraCT database (REFNO 2020-001595-15). Results will be presented in peer-reviewed journals, at meetings and at international conferences.
Keywords: adult nephrology; chronic renal failure; diabetic nephropathy & vascular disease; nephrology.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous