Effects of cascade reporting of susceptibility profiles for Enterobacterales on broad-spectrum antibiotics use and resistance
- PMID: 35190452
- PMCID: PMC8899679
- DOI: 10.1136/ejhpharm-2021-002951
Effects of cascade reporting of susceptibility profiles for Enterobacterales on broad-spectrum antibiotics use and resistance
Abstract
Objective: To reduce the inappropriate use of broad-spectrum antibiotics in a 1000+ bed acute tertiary care hospital by the introduction of cascade antimicrobial susceptibility reporting for Enterobacterales.
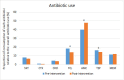
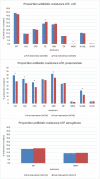
Methods: Over a 1-year period, we selectively suppressed reporting of susceptibility to the broad-spectrum antibiotics piperacillin-tazobactam (TZP) and meropenem (MEM) for Enterobacterales strains susceptible to amoxicillin-clavulanic acid (AMC) and negative for extended-spectrum β-lactamase (ESBL). We measured the effects on hospital-wide antibiotic consumption (defined daily doses/1000 admissions) and resistance of Escherichia coli and Klebsiella pneumoniae on two levels. First, we compared resistance and antibiotic use for the antibiotics impacted by the intervention (AMC, TZP and MEM) with control antibiotics that were consistently reported (fluoroquinolones, trimethoprim-sulfamethoxazole and third-generation cephalosporins). Second, we compared the resistance for TZP and MEM with a control pathogen (Pseudomonas aeruginosa) and studied the impact on rate of Clostridioides difficile-associated diarrhoea in our hospital.
Results: We observed an overall increased use of AMC relative to overall antibiotic consumption (20.0%, p<0.0001) together with a decreased use of TZP (-11.9%, p=0.049) and unchanged use of MEM (p=0.68) relative to overall antibiotic consumption. As for resistance, the number of ESBL-positive K. pneumoniae strains diminished by 5.9% (p<0.0001). When focusing on intensive care units, the carbapenemase-producing Enterobacterales (CPE) rate also decreased by 4.5% (p=0.0091). For E. coli, no significant difference in ESBL (p=0.33) and CPE (p=0.48) rates were observed. No significant difference in the rate of C. difficile infections was observed (p=0.40).
Conclusions: Restricted susceptibility reporting of TZP and MEM was associated with a significant increased use of AMC and decreased use of TZP relative to overall antibiotic consumption and significant reduction in ESBL- and CPE-positive K. pneumoniae strains.
Keywords: clinical laboratory techniques; clinical medicine; hospital; laboratories; microbiological techniques; microbiology.
© European Association of Hospital Pharmacists 2022. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JDW: grant from the Flanders Research Foundation during the conduct of the study (Senior Clinical Investigator Grant); consulted for Accelerate, Bayer Healthcare, Cubist, Grifols, MSD and Pfizer (honoraria were paid to his institution).
Figures
Similar articles
-
[Evolution of susceptibility to antibiotics of Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumanii, in a University Hospital Center of Beirut between 2005 and 2009].Pathol Biol (Paris). 2012 Jun;60(3):e15-20. doi: 10.1016/j.patbio.2011.03.011. Epub 2011 Jun 29. Pathol Biol (Paris). 2012. PMID: 21719212 French.
-
Diminished Susceptibility to Cefoperazone/Sulbactam and Piperacillin/Tazobactam in Enterobacteriaceae Due to Narrow-Spectrum β-Lactamases as Well as Omp Mutation.Pol J Microbiol. 2022 Jun 19;71(2):251-256. doi: 10.33073/pjm-2022-023. Pol J Microbiol. 2022. PMID: 35716168 Free PMC article.
-
Update of incidence and antimicrobial susceptibility trends of Escherichia coli and Klebsiella pneumoniae isolates from Chinese intra-abdominal infection patients.BMC Infect Dis. 2017 Dec 18;17(1):776. doi: 10.1186/s12879-017-2873-z. BMC Infect Dis. 2017. PMID: 29254478 Free PMC article.
-
High prevalence of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolates: A 5-year retrospective study at a Tertiary Hospital in Northern Thailand.Front Cell Infect Microbiol. 2022 Aug 8;12:955774. doi: 10.3389/fcimb.2022.955774. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36004324 Free PMC article.
-
Sulopenem: An Intravenous and Oral Penem for the Treatment of Urinary Tract Infections Due to Multidrug-Resistant Bacteria.Drugs. 2022 Apr;82(5):533-557. doi: 10.1007/s40265-022-01688-1. Epub 2022 Mar 16. Drugs. 2022. PMID: 35294769 Review.
Cited by
-
Point-Counterpoint: Cascade reporting-useful tool to support antimicrobial stewardship, or dangerously misleading.J Clin Microbiol. 2025 Jul 9;63(7):e0170824. doi: 10.1128/jcm.01708-24. Epub 2025 Jun 17. J Clin Microbiol. 2025. PMID: 40526395 Free PMC article. Review.
-
Short report: impact of selective reporting of antibiotic susceptibility testing on antibiotic use in patients with bloodstream infection with Enterococcus faecalis.Infection. 2023 Oct;51(5):1557-1562. doi: 10.1007/s15010-023-02045-4. Epub 2023 May 23. Infection. 2023. PMID: 37217812
-
Impact of Selective Reporting of Antibiotic Susceptibility Test Results on Antibiotic Use in Patients with Bloodstream Infection with Streptococcus pneumoniae.Biomed Hub. 2024 May 20;9(1):67-72. doi: 10.1159/000537770. eCollection 2024 Jan-Dec. Biomed Hub. 2024. PMID: 39015199 Free PMC article.
References
-
- Magiorakos AP, Burns K, Rodríguez Baño J, et al. . Infection prevention and control measures and tools for the prevention of entry of carbapenem-resistant Enterobacteriaceae into healthcare settings: guidance from the European Centre for Disease Prevention and Control. Antimicrob Resist Infect Control 2017;6:113. 10.1186/s13756-017-0259-z - DOI - PMC - PubMed
-
- Stainton SM, Thabit AK, Kuti JL, et al. . Prevalence, patient characteristics and outcomes of a novel piperacillin/tazobactam-resistant, pan-β-lactam-susceptible phenotype in Enterobacteriaceae: implications for selective reporting. Clin Microbiol Infect 2017;23:581–2. 10.1016/j.cmi.2017.02.012 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
