Heat inactivation of clinical COVID-19 samples on an industrial scale for low risk and efficient high-throughput qRT-PCR diagnostic testing
- PMID: 35190592
- PMCID: PMC8861189
- DOI: 10.1038/s41598-022-06888-z
Heat inactivation of clinical COVID-19 samples on an industrial scale for low risk and efficient high-throughput qRT-PCR diagnostic testing
Abstract
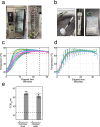
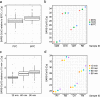
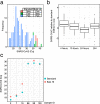
We report the development of a large scale process for heat inactivation of clinical COVID-19 samples prior to laboratory processing for detection of SARS-CoV-2 by RT-qPCR. With more than 266 million confirmed cases, over 5.26 million deaths already recorded at the time of writing, COVID-19 continues to spread in many parts of the world. Consequently, mass testing for SARS-CoV-2 will remain at the forefront of the COVID-19 response and prevention for the near future. Due to biosafety considerations the standard testing process requires a significant amount of manual handling of patient samples within calibrated microbiological safety cabinets. This makes the process expensive, effects operator ergonomics and restricts testing to higher containment level laboratories. We have successfully modified the process by using industrial catering ovens for bulk heat inactivation of oropharyngeal/nasopharyngeal swab samples within their secondary containment packaging before processing in the lab to enable all subsequent activities to be performed in the open laboratory. As part of a validation process, we tested greater than 1200 clinical COVID-19 samples and showed less than 1 Cq loss in RT-qPCR test sensitivity. We also demonstrate the bulk heat inactivation protocol inactivates a murine surrogate of human SARS-CoV-2. Using bulk heat inactivation, the assay is no longer reliant on containment level 2 facilities and practices, which reduces cost, improves operator safety and ergonomics and makes the process scalable. In addition, heating as the sole method of virus inactivation is ideally suited to streamlined and more rapid workflows such as 'direct to PCR' assays that do not involve RNA extraction or chemical neutralisation methods.
© 2022. The Author(s).
Conflict of interest statement
Colin Barker is Chief Scientific Officer for BiologIC Technologies. All other authors declare no competing interests relevant to the submitted work.
Figures
Similar articles
-
Comparative effects of viral-transport-medium heat inactivation upon downstream SARS-CoV-2 detection in patient samples.J Med Microbiol. 2021 Mar;70(3):001301. doi: 10.1099/jmm.0.001301. Epub 2021 Mar 18. J Med Microbiol. 2021. PMID: 33734960 Free PMC article.
-
Resilient SARS-CoV-2 diagnostics workflows including viral heat inactivation.PLoS One. 2021 Sep 15;16(9):e0256813. doi: 10.1371/journal.pone.0256813. eCollection 2021. PLoS One. 2021. PMID: 34525109 Free PMC article.
-
Extraction-free protocol combining proteinase K and heat inactivation for detection of SARS-CoV-2 by RT-qPCR.PLoS One. 2021 Feb 26;16(2):e0247792. doi: 10.1371/journal.pone.0247792. eCollection 2021. PLoS One. 2021. PMID: 33635936 Free PMC article.
-
Detection profile of SARS-CoV-2 using RT-PCR in different types of clinical specimens: A systematic review and meta-analysis.J Med Virol. 2021 Feb;93(2):719-725. doi: 10.1002/jmv.26349. Epub 2020 Aug 2. J Med Virol. 2021. PMID: 32706393 Free PMC article.
-
β-Propiolactone (BPL)-inactivation of SARS-Co-V-2: In vitro validation with focus on saliva from COVID-19 patients for scent dog training.J Virol Methods. 2023 Jul;317:114733. doi: 10.1016/j.jviromet.2023.114733. Epub 2023 Apr 15. J Virol Methods. 2023. PMID: 37068591 Free PMC article. Review.
Cited by
-
Rapid and reliable inactivation protocols for the diagnostics of emerging viruses: The example of SARS-CoV-2 and monkeypox virus.J Med Virol. 2023 Jan;95(1):e28126. doi: 10.1002/jmv.28126. Epub 2022 Sep 21. J Med Virol. 2023. PMID: 36089749 Free PMC article.
-
High-Throughput COVID-19 Testing of Naso-Oropharyngeal Swabs Using a Sensitive Extraction-Free Sample Preparation Method.Microbiol Spectr. 2022 Aug 31;10(4):e0135822. doi: 10.1128/spectrum.01358-22. Epub 2022 Aug 11. Microbiol Spectr. 2022. PMID: 35950846 Free PMC article.
-
Rapid assays of SARS-CoV-2 virus and noble biosensors by nanomaterials.Nano Converg. 2024 Jan 8;11(1):2. doi: 10.1186/s40580-023-00408-z. Nano Converg. 2024. PMID: 38190075 Free PMC article. Review.
-
Current status of pathogen handling in European laboratories: focus on viral inactivation process.Front Bioeng Biotechnol. 2024 Jun 7;12:1422553. doi: 10.3389/fbioe.2024.1422553. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38911551 Free PMC article.
-
Rapid inactivation and sample preparation for SARS-CoV-2 PCR-based diagnostics using TNA-Cifer Reagent E.Front Microbiol. 2023 Oct 6;14:1238542. doi: 10.3389/fmicb.2023.1238542. eCollection 2023. Front Microbiol. 2023. PMID: 37869655 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

