Half of germline pathogenic and likely pathogenic variants found on panel tests do not fulfil NHS testing criteria
- PMID: 35190596
- PMCID: PMC8861039
- DOI: 10.1038/s41598-022-06376-4
Half of germline pathogenic and likely pathogenic variants found on panel tests do not fulfil NHS testing criteria
Abstract
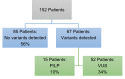
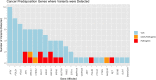
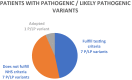
Genetic testing for cancer predisposition has been curtailed by the cost of sequencing, and testing has been restricted by eligibility criteria. As the cost of sequencing decreases, the question of expanding multi-gene cancer panels to a broader population arises. We evaluated how many additional actionable genetic variants are returned by unrestricted panel testing in the private sector compared to those which would be returned by adhering to current NHS eligibility criteria. We reviewed 152 patients referred for multi-gene cancer panels in the private sector between 2014 and 2016. Genetic counselling and disclosure of all results was standard of care provided by the Consultant. Every panel conducted was compared to current eligibility criteria. A germline pathogenic / likely pathogenic variant (P/LP), in a gene relevant to the personal or family history of cancer, was detected in 15 patients (detection rate of 10%). 46.7% of those found to have the P/LP variants (7 of 15), or 4.6% of the entire set (7 of 152), did not fulfil NHS eligibility criteria. 46.7% of P/LP variants in this study would have been missed by national testing guidelines, all of which were actionable. However, patients who do not fulfil eligibility criteria have a higher Variant of Uncertain Significance (VUS) burden. We demonstrated that the current England NHS threshold for genetic testing is missing pathogenic variants which would alter management in 4.6%, nearly 1 in 20 individuals. However, the clinical service burden that would ensue is a detection of VUS of 34%.
© 2022. The Author(s).
Conflict of interest statement
Professor Rosalind Eeles: 1. GU-ASCO meeting in San Francisco - Jan 2016 – Honorarium as speaker $500 2. RMH FR meeting – Nov 2017 – support from Janssen, honorarium as speaker £1100 (Title: Genetics and Prostate Cancer) 3. University of Chicago invited talk May 2018 – Honorarium as speaker $1000 4. EUR 200 educational honorarium paid by Bayer & Ipsen to attend GU Connect “Treatment sequencing for mCRPC patients within the changing landscape of mHSPC” at a venue at ESMO, Barcelona, 28 September 2019 5. Prostate Dx Advisory Panel – Member of external Expert Committee. 30th June 2002/3 hours/ £900. The remaining authors have no conflicts of interest to declare.
Figures




References
-
- Taylor, A., Brady, A. F,, Frayling, I. M., Hanson, H., Tischkowitz, M., Turnbull, C., Side, L. & Group UKCG. Consensus for genes to be included on cancer panel tests offered by UK genetics services: Guidelines of the UK Cancer Genetics Group. J. Med. Genet.16, 16. 10.1136/jmedgenet-2017-105188 (2018). - PMC - PubMed
-
- Whitworth J, Smith PS, Martin JE, West H, Luchetti A, Rodger F, Clark G, Carss K, Stephens J, Stirrups K, Penkett C, Mapeta R, Ashford S, Megy K, Shakeel H, Ahmed M, Adlard J, Barwell J, Brewer C, Casey RT, Armstrong R, Cole T, Evans DG, Fostira F, Greenhalgh L, Hanson H, Henderson A, Hoffman J, Izatt L, Kumar A, Kwong A, Lalloo F, Ong KR, Paterson J, Park SM, Chen-Shtoyerman R, Searle C, Side L, Skytte AB, Snape K, Woodward ER, Consortium NBRD, Tischkowitz MD, Maher ER. Comprehensive cancer-predisposition gene testing in an adult multiple primary tumor series shows a broad range of deleterious variants and atypical tumor phenotypes. Am. J. Human Genet. 2018;12:12. doi: 10.1016/j.ajhg.2018.04.013. - DOI - PMC - PubMed
-
- National Collaborating Centre for Cancer (UK). Familial Breast Cancer: Classification and Care of People at Risk of Familial Breast Cancer and Management of Breast Cancer and Related Risks in People with a Family History of Breast Cancer. Cardiff (UK): National Collaborating Centre for Cancer (UK); 2013 Jun. (NICE Clinical Guidelines, No. 164.) Available from: https://www.ncbi.nlm.nih.gov/books/NBK247567/ - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

