Future perspectives of uveal melanoma blood based biomarkers
- PMID: 35190695
- PMCID: PMC9130512
- DOI: 10.1038/s41416-022-01723-8
Future perspectives of uveal melanoma blood based biomarkers
Abstract
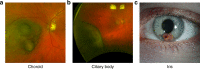
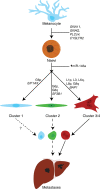
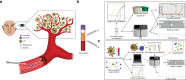
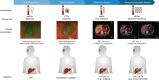
Uveal melanoma (UM) is the most common primary intraocular malignancy affecting adults. Despite successful local treatment of the primary tumour, metastatic disease develops in up to 50% of patients. Metastatic UM carries a particularly poor prognosis, with no effective therapeutic option available to date. Genetic studies of UM have demonstrated that cytogenetic features, including gene expression, somatic copy number alterations and specific gene mutations can allow more accurate assessment of metastatic risk. Pre-emptive therapies to avert metastasis are being tested in clinical trials in patients with high-risk UM. However, current prognostic methods require an intraocular tumour biopsy, which is a highly invasive procedure carrying a risk of vision-threatening complications and is limited by sampling variability. Recently, a new diagnostic concept known as "liquid biopsy" has emerged, heralding a substantial potential for minimally invasive genetic characterisation of tumours. Here, we examine the current evidence supporting the potential of blood circulating tumour cells (CTCs), circulating tumour DNA (ctDNA), microRNA (miRNA) and exosomes as biomarkers for UM. In particular, we discuss the potential of these biomarkers to aid clinical decision making throughout the management of UM patients.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Dogrusoz M, Jager MJ, Damato B. Uveal melanoma treatment and prognostication. Asia Pac J Ophthalmol (Philos) 2017;6:186–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

