Sepsis with liver dysfunction and coagulopathy predicts an inflammatory pattern of macrophage activation
- PMID: 35190900
- PMCID: PMC8861227
- DOI: 10.1186/s40635-022-00433-y
Sepsis with liver dysfunction and coagulopathy predicts an inflammatory pattern of macrophage activation
Abstract
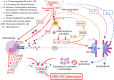
Background: Interleukin-1 receptor antagonists can reduce mortality in septic shock patients with hepatobiliary dysfunction and disseminated intravascular coagulation (HBD + DIC), an organ failure pattern with inflammatory features consistent with macrophage activation. Identification of clinical phenotypes in sepsis may allow for improved care. We aim to describe the occurrence of HBD + DIC in a contemporary cohort of patients with sepsis and determine the association of this phenotype with known macrophage activation syndrome (MAS) biomarkers and mortality. We performed a retrospective nested case-control study in adult septic shock patients with concurrent HBD + DIC and an equal number of age-matched controls, with comparative analyses of all-cause mortality and circulating biomarkers between the groups. Multiple logistic regression explored the effect of HBD + DIC on mortality and the discriminatory power of the measured biomarkers for HBD + DIC and mortality.
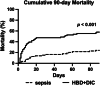
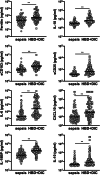
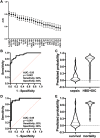
Results: Six percent of septic shock patients (n = 82/1341) had HBD + DIC, which was an independent risk factor for 90-day mortality (OR = 3.1, 95% CI 1.4-7.5, p = 0.008). Relative to sepsis controls, the HBD + DIC cohort had increased levels of 21 of the 26 biomarkers related to macrophage activation (p < 0.05). This panel was predictive of both HBD + DIC (sensitivity = 82%, specificity = 84%) and mortality (sensitivity = 92%, specificity = 90%).
Conclusion: The HBD + DIC phenotype identified patients with high mortality and a molecular signature resembling that of MAS. These observations suggest trials of MAS-directed therapies are warranted.
Keywords: Ferritin; IL-18; Organ dysfunction; Phenotype; Sepsis.
© 2022. The Author(s).
Conflict of interest statement
RRA reports stipend support from The American Association of Immunologists; HG, DCA, DTH, and JAK report funding from the National Institutes of Health; SWC reports funding from the National Institutes of Health, the RK Mellon Institute for Pediatric Research, research support unrelated to the current work from AB2Bio and IMMvention therapeutix; DMY reports funding from the National Institutes of Health, research support unrelated to the current work from the National Institutes of Health, royalties from Wolters/Kluwer, Lippincott/Williams and Wilkins, UpToDate Inc, and McGraw Hill Inc., editorial stipend from the American College of Emergency Physicians, payment for expert consultation from multiple legal firms, honorarium for participation on an advisory board with University of Pennsylvania, payment from National Quality Forum committees, and payment from the PA Board of Medicine; JAC reports funding from the National Institutes of Health for research support both related and unrelated to the current work. None of the remaining authors report any disclosures.
Figures
References
-
- Ravelli A, Magni-Manzoni S, Pistorio A, Besana C, Foti T, Ruperto N, Viola S, Martini A. Preliminary diagnostic guidelines for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. J Pediatr. 2005;146:598–604. - PubMed
-
- Fardet L, Galicier L, Lambotte O, Marzac C, Aumont C, Chahwan D, Coppo P, Hejblum G. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66:2613–2620. - PubMed
-
- Ravelli A, Minoia F, Davi S, Horne A, Bovis F, Pistorio A, Arico M, Avcin T, Behrens EM, De Benedetti F, Filipovic L, Grom AA, Henter JI, Ilowite NT, Jordan MB, Khubchandani R, Kitoh T, Lehmberg K, Lovell DJ, Miettunen P, Nichols KE, Ozen S, Schmid PJ, Ramanan AV, Russo R, Schneider R, Sterba G, Uziel Y, Wallace C, Wouters C, Wulffraat N, Demirkaya E, Brunner HI, Martini A, Ruperto N, Cron RQ, Paediatric Rheumatology International Trials O. Childhood A, Rheumatology Research A, Pediatric Rheumatology Collaborative Study G. Histiocyte S. 2016 classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Arthritis Rheumatol. 2016;68:566–576. - PubMed

