Background rates of five thrombosis with thrombocytopenia syndromes of special interest for COVID-19 vaccine safety surveillance: Incidence between 2017 and 2019 and patient profiles from 38.6 million people in six European countries
- PMID: 35191114
- PMCID: PMC9088543
- DOI: 10.1002/pds.5419
Background rates of five thrombosis with thrombocytopenia syndromes of special interest for COVID-19 vaccine safety surveillance: Incidence between 2017 and 2019 and patient profiles from 38.6 million people in six European countries
Abstract
Background: Thrombosis with thrombocytopenia syndrome (TTS) has been reported among individuals vaccinated with adenovirus-vectored COVID-19 vaccines. In this study, we describe the background incidence of non-vaccine induced TTS in six European countries.
Methods: Electronic medical records from France, the Netherlands, Italy, Germany, Spain, and the United Kingdom informed the study. Incidence rates of cerebral venous sinus thrombosis (CVST), splanchnic vein thrombosis (SVT), deep vein thrombosis (DVT), pulmonary embolism (PE), and myocardial infarction or ischemic stroke, all with concurrent thrombocytopenia, were estimated among the general population of persons in a database between 2017 and 2019. A range of additional potential adverse events of special interest for COVID-19 vaccinations were also studied in a similar manner.
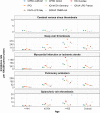
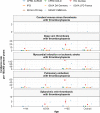
Findings: A total of 38 611 617 individuals were included. Background rates ranged from 1.0 (95% CI: 0.7-1.4) to 8.5 (7.4-9.9) per 100 000 person-years for DVT with thrombocytopenia, from 0.5 (0.3-0.6) to 20.8 (18.9-22.8) for PE with thrombocytopenia, from 0.1 (0.0-0.1) to 2.5 (2.2-2.7) for SVT with thrombocytopenia, and from 1.0 (0.8-1.2) to 43.4 (40.7-46.3) for myocardial infarction or ischemic stroke with thrombocytopenia. CVST with thrombocytopenia was only identified in one database, with incidence rate of 0.1 (0.1-0.2) per 100 000 person-years. The incidence of non-vaccine induced TTS increased with age, and was typically greater among those with more comorbidities and greater medication use than the general population. It was also more often seen in men than women. A large proportion of those affected were seen to have been taking antithrombotic and anticoagulant therapies prior to their event.
Interpretation: Although rates vary across databases, non-vaccine induced TTS has consistently been seen to be a very rare event among the general population. While still remaining very rare, rates were typically higher among older individuals, and those affected were also seen to generally be male and have more comorbidities and greater medication use than the general population.
Keywords: Covid-19; post vaccine; thrombosis-thrombocytopenia syndromes (TTS); vaccine.
Conflict of interest statement
DPA's research group has received research grants from the European Medicines Agency, from the Innovative Medicines Initiative, from Amgen, Chiesi, and from UCB Biopharma; and consultancy or speaker fees from Astellas, Amgen and UCB Biopharma. At the time of analysis, Kristin Kostka, Henry Morgan Stewart, Carlen Reyes and Sarah Seager were employees of IQVIA. Kristin Kostka reported receiving funding from the National Institutes of Health National COVID Cohort Collaborative (N3C). IQVIA received funding from the University of Oxford on behalf of the Bill & Melinda Gates Foundation for the conversion of LPD Italy and utilisation of DA Germany data for COVID‐19 related research. Katia Verhamme and Peter Rijnbeek work for a research group that received unconditional research grants from Yamanouchi, Pfizer/Boehringer Ingelheim, Novartis, GSK, Amgen, Astra‐Zeneca, UCB, J&J, the European Medicines Agency and the Innovative Medicines Initiative.
Figures


References
-
- Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99‐111. doi: 10.1016/S0140-6736(20)32661-1 - DOI - PMC - PubMed
-
- Nature . ‘Unprecedented achievement’: who received the first billion COVID vaccinations? 2021. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

