Asymmetric Synthesis of Axially Chiral C-N Atropisomers
- PMID: 35191558
- PMCID: PMC9314733
- DOI: 10.1002/chem.202104442
Asymmetric Synthesis of Axially Chiral C-N Atropisomers
Abstract
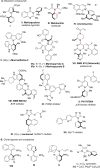
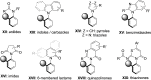
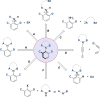
Molecules with restricted rotation around a single bond or atropisomers are found in a wide number of natural products and bioactive molecules as well as in chiral ligands for asymmetric catalysis and smart materials. Although most of these compounds are biaryls and heterobiaryls displaying a C-C stereogenic axis, there is a growing interest in less common and more challenging axially chiral C-N atropisomers. This review offers an overview of the various methodologies available for their asymmetric synthesis. A brief introduction is initially given to contextualize these axially chiral skeletons, including a historical background and examples of natural products containing axially chiral C-N axes. The preparation of different families of C-N based atropisomers is then presented from anilides to chiral five- and six-membered ring heterocycles. Special emphasis has been given to modern catalytic asymmetric strategies over the past decade for the synthesis of these chiral scaffolds. Applications of these methods to the preparation of natural products and biologically active molecules will be highlighted along the text.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

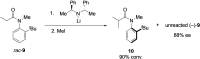
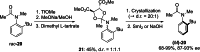
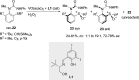


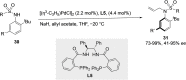
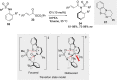
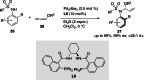


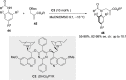
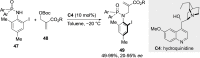

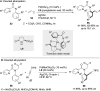
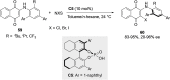
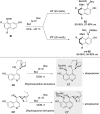
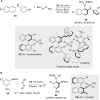

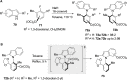
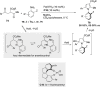

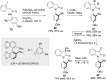
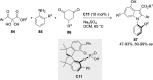

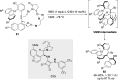
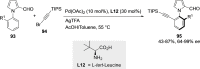
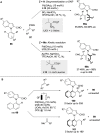

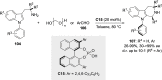
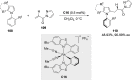
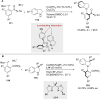
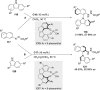
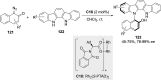
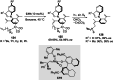
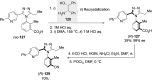
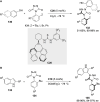
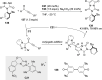
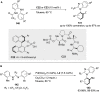
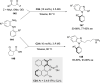
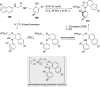



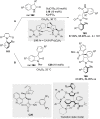
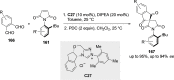

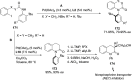
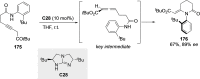
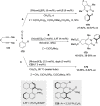

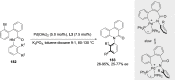
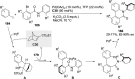
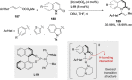

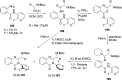
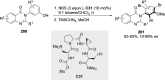

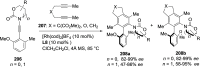

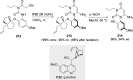
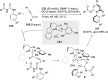
References
-
- Oki M., Top. Stereochem. 1983, 14, 1–81.
-
- Christie G. H., Kenner J., J. Chem. Soc. Trans. 1922, 120, 614–620.
-
- Noyori R., Angew. Chem. Int. Ed. 2002, 41, 2008–2022; - PubMed
- Angew. Chem. 2002, 114, 2108–2123.
-
- None
-
- Zhang D., Wang Q., Coord. Chem. Rev. 2015, 286, 1–16;
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

