Expanding the Repertoire of Low-Molecular-Weight Pentafluorosulfanyl-Substituted Scaffolds
- PMID: 35191598
- PMCID: PMC9305131
- DOI: 10.1002/cmdc.202100641
Expanding the Repertoire of Low-Molecular-Weight Pentafluorosulfanyl-Substituted Scaffolds
Abstract
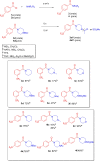
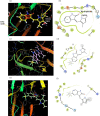
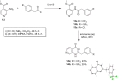
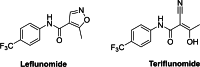
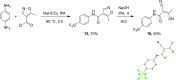
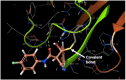
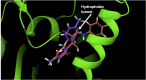
The pentafluorosulfanyl (-SF5 ) functional group is of increasing interest as a bioisostere in medicinal chemistry. A library of SF5 -containing compounds, including amide, isoxazole, and oxindole derivatives, was synthesised using a range of solution-based and solventless methods, including microwave and ball-mill techniques. The library was tested against targets including human dihydroorotate dehydrogenase (HDHODH). A subsequent focused approach led to synthesis of analogues of the clinically used disease modifying anti-rheumatic drugs (DMARDs), Teriflunomide and Leflunomide, considered for potential COVID-19 use, where SF5 bioisostere deployment led to improved inhibition of HDHODH compared with the parent drugs. The results demonstrate the utility of the SF5 group in medicinal chemistry.
Keywords: COVID-19, SARS-COV-2 main protease (Mpro); DMARDs; SF5 group.
© 2022 The Authors. ChemMedChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


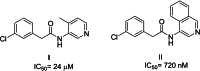


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

